ODYSSEY clinical trial design: a randomised global study to evaluate the efficacy and safety of dolutegravir-based antiretroviral therapy in HIV-positive children, with nested pharmacokinetic sub-studies to evaluate pragmatic WHO-weight-band based dolutegravir dosing
- PMID: 33446115
- PMCID: PMC7809782
- DOI: 10.1186/s12879-020-05672-6
ODYSSEY clinical trial design: a randomised global study to evaluate the efficacy and safety of dolutegravir-based antiretroviral therapy in HIV-positive children, with nested pharmacokinetic sub-studies to evaluate pragmatic WHO-weight-band based dolutegravir dosing
Abstract
Background: Dolutegravir (DTG)-based antiretroviral therapy (ART) is highly effective and well-tolerated in adults and is rapidly being adopted globally. We describe the design of the ODYSSEY trial which evaluates the efficacy and safety of DTG-based ART compared with standard-of-care in children and adolescents. The ODYSSEY trial includes nested pharmacokinetic (PK) sub-studies which evaluated pragmatic World Health Organization (WHO) weight-band-based DTG dosing and opened recruitment to children < 14 kg while dosing was in development.
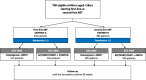
Methods: ODYSSEY (Once-daily DTG based ART in Young people vS. Standard thErapY) is an open-label, randomised, non-inferiority, basket trial comparing the efficacy and safety of DTG + 2 nucleos(t) ides (NRTIs) versus standard-of-care (SOC) in HIV-infected children < 18 years starting first-line ART (ODYSSEY A) or switching to second-line ART (ODYSSEY B). The primary endpoint is clinical or virological failure by 96 weeks.
Results: Between September 2016 and June 2018, 707 children weighing ≥14 kg were enrolled; including 311 ART-naïve children and 396 children starting second-line. 47% of children were enrolled in Uganda, 21% Zimbabwe, 20% South Africa, 9% Thailand, 4% Europe. 362 (51%) participants were male; median age [range] at enrolment was 12.2 years [2.9-18.0]. 82 (12%) children weighed 14 to < 20 kg, 135 (19%) 20 to < 25 kg, 206 (29%) 25 to < 35 kg, 284 (40%) ≥35 kg. 128 (18%) had WHO stage 3 and 60 (8%) WHO stage 4 disease. Challenges encountered include: (i) running the trial across high- to low-income countries with differing frequencies of standard-of-care viral load monitoring; (ii) evaluating pragmatic DTG dosing in PK sub-studies alongside FDA- and EMA-approved dosing and subsequently transitioning participants to new recommended doses; (iii) delays in dosing information for children weighing 3 to < 14 kg and rapid recruitment of ART-naïve older/heavier children, which led to capping recruitment of participants weighing ≥35 kg in ODYSSEY A and extending recruitment (above 700) to allow for ≥60 additional children weighing between 3 to < 14 kg with associated PK; (iv) a safety alert associated with DTG use during pregnancy, which required a review of the safety plan for adolescent girls.
Conclusions: By employing a basket design, to include ART-naïve and -experienced children, and nested PK sub-studies, the ODYSSEY trial efficiently evaluates multiple scientific questions regarding dosing and effectiveness of DTG-based ART in children.
Trial registration: NCT, NCT02259127 , registered 7th October 2014; EUDRACT, 2014-002632-14, registered 18th June 2014 ( https://www.clinicaltrialsregister.eu/ctr-search/trial/2014-002632-14/ES ); ISRCTN, ISRCTN91737921 , registered 4th October 2014.
Keywords: Basket trial; Dolutegravir; Efficacy; HIV; Paediatric; Pharmacokinetic; Randomized control trial; Safety.
Conflict of interest statement
MFC is a member of an advisory board for ViiV Healthcare and reports grants from ViiV Healthcare and Gilead, outside the submitted work. DMB is a member of an advisory board for Merck and ViiV Healthcare and reports grants from Merck, BMS, Janssen/Tibotec, Gilead and ViiV Healthcare outside the submitted work. All other authors declare that they have no competing interests.
Figures
References
-
- World Health Organization (WHO) Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Geneva: WHO; 2016. - PubMed
-
- UNAIDS . Aids by the Numbers. 2016.
-
- UNAIDS . Children and HIV. 2016.
-
- Clinton Health Access Initiative (CHAI) ARV Market Report: The state of the antiretroviral drug market in low- and middle-income countries, 2016–2021. 2017.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

