Hydroxychloroquine in the treatment of outpatients with mildly symptomatic COVID-19: a multi-center observational study
- PMID: 33446136
- PMCID: PMC7807228
- DOI: 10.1186/s12879-021-05773-w
Hydroxychloroquine in the treatment of outpatients with mildly symptomatic COVID-19: a multi-center observational study
Abstract
Background: Hydroxychloroquine has not been associated with improved survival among hospitalized COVID-19 patients in the majority of observational studies and similarly was not identified as an effective prophylaxis following exposure in a prospective randomized trial. We aimed to explore the role of hydroxychloroquine therapy in mildly symptomatic patients diagnosed in the outpatient setting.
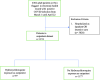
Methods: We examined the association between outpatient hydroxychloroquine exposure and the subsequent progression of disease among mildly symptomatic non-hospitalized patients with documented SARS-CoV-2 infection. The primary outcome assessed was requirement of hospitalization. Data was obtained from a retrospective review of electronic health records within a New Jersey USA multi-hospital network. We compared outcomes in patients who received hydroxychloroquine with those who did not applying a multivariable logistic model with propensity matching.
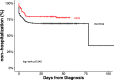
Results: Among 1274 outpatients with documented SARS-CoV-2 infection 7.6% were prescribed hydroxychloroquine. In a 1067 patient propensity matched cohort, 21.6% with outpatient exposure to hydroxychloroquine were hospitalized, and 31.4% without exposure were hospitalized. In the primary multivariable logistic regression analysis with propensity matching there was an association between exposure to hydroxychloroquine and a decreased rate of hospitalization from COVID-19 (OR 0.53; 95% CI, 0.29, 0.95). Sensitivity analyses revealed similar associations. QTc prolongation events occurred in 2% of patients prescribed hydroxychloroquine with no reported arrhythmia events among those with data available.
Conclusions: In this retrospective observational study of SARS-CoV-2 infected non-hospitalized patients hydroxychloroquine exposure was associated with a decreased rate of subsequent hospitalization. Additional exploration of hydroxychloroquine in this mildly symptomatic outpatient population is warranted.
Keywords: COVID-19; Hydroxychloroquine; Outpatient.
Conflict of interest statement
Figures
References
-
- Pan H, Peto R, Karim QA, Alejandria M, Henao-Restrepo AM, Garcia CH, et al. Repurposed antiviral drugs for COVID-19 -interim WHO SOLIDARITY trial results. medRxiv. 2020. 10.1101/2020.10.15.20209817.
-
- New Jersey COVID-19 Information Hub. https://covid19.nj.gov/. Accessed 18 June 2020.
-
- Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covidview/index.html. Accessed 27 Nov 2020.
-
- Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020. 10.1016/j.ijantimicag.2020.105949 [published online ahead of print, 2020 Mar 20]. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

