Safety and feasibility of apheresis to harvest and concentrate parasites from subjects with induced blood stage Plasmodium vivax infection
- PMID: 33446191
- PMCID: PMC7807416
- DOI: 10.1186/s12936-021-03581-w
Safety and feasibility of apheresis to harvest and concentrate parasites from subjects with induced blood stage Plasmodium vivax infection
Abstract
Background: In the absence of a method to culture Plasmodium vivax, the only way to source parasites is ex vivo. This hampers many aspects of P. vivax research. This study aimed to assess the safety of apheresis, a method for selective removal of specific components of blood as a means of extracting and concentrating P. vivax parasites.
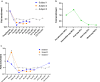
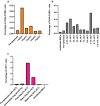
Methods: An iterative approach was employed across four non-immune healthy human subjects in single subject cohorts. All four subjects were inoculated with ~ 564 blood stage P. vivax (HMP013-Pv) and subjected to apheresis 10 to 11 days later. Blood samples collected during apheresis (haematocrit layers 0.5% to 11%) were tested for the presence and concentration of P. vivax by microscopy, flow cytometry, 18S rDNA qPCR for total parasites, and pvs25 qRT-PCR for female gametocyte transcripts. Safety was determined by monitoring adverse events. Malaria transmission to mosquitoes was assessed by membrane feeding assays.
Results: There were no serious adverse events and no significant safety concerns. Apheresis concentrated asexual parasites by up to 4.9-fold (range: 0.9-4.9-fold) and gametocytes by up to 1.45-fold (range: 0.38-1.45-fold) compared to pre-apheresis densities. No single haematocrit layer contained > 40% of all the recovered P. vivax asexual parasites. Ex vivo concentration of parasites by Percoll gradient centrifugation of whole blood achieved greater concentration of gametocytes than apheresis. Mosquito transmission was enhanced by up to fivefold in a single apheresis sample compared to pre-apheresis.
Conclusion: The modest level of parasite concentration suggests that the use of apheresis may not be an ideal method for harvesting P. vivax. Trial Registration Australia New Zealand Clinical Trials Registry (ANZCTR) Trial ID: ACTRN12617001502325 registered on 19th October 2017. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=373812.
Keywords: Apheresis; Concentration; Malaria; Parasite; Plasmodium.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- WHO. World Malaria Report 2019. Geneva, World Health Organization; 2019.
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

