AYUSH 64, a polyherbal Ayurvedic formulation in Influenza-like illness - Results of a pilot study
- PMID: 33446377
- PMCID: PMC8718941
- DOI: 10.1016/j.jaim.2020.05.010
AYUSH 64, a polyherbal Ayurvedic formulation in Influenza-like illness - Results of a pilot study
Abstract
Background: Influenza-like Illness (ILI) refers to a wide range of viral infections with an important cause of morbidity and mortality worldwide. The global incidence of ILI is estimated at 5-10% in adults and 20-30% in children. In India influenza accounts for 20-42% of monthly acute medical illness hospitalizations during the peak rainy season. AYUSH-64, a poly-herbal drug, is in practice for 40 years for various clinical conditions like fevers, microfilaremia, and inflammatory conditions.
Objective: A pilot study was conducted to evaluate the safety and efficacy of Ayurvedic formulation, AYUSH-64 in clinically diagnosed ILI for accelerating the recovery.
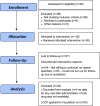
Material and methods: A prospective, open-label, nonrandomized, single group, single-center pilot clinical study with pre-test and post-test design was conducted at Raja Ramdeo Anandilal Podar Central Ayurveda Research Institute for Cancer, Mumbai, an institute of Central Council for Research in Ayurvedic Sciences (CCRAS) between June 2018 and July 2019. A total of 38 participants of clinically diagnosed ILI (18-65 years) were studied with an one-week intervention of 'AYUSH 64' in a dose of 3 gm/day and three weeks post-treatment observation period. Assessment of parameters viz. improvement in the symptoms of ILI, frequency of usage of acetaminophen, antihistaminic and cough syrup, hematology, liver function and kidney function tests along with incidence of secondary complications, and time to return to a normal routine was done.
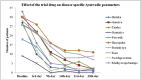
Results: One-week intervention of AYUSH 64 helped to recover from ILI symptoms with reduced frequency of usage of acetaminophen and antihistaminic. The intervention was safe on hematology and biochemical parameters. No serious adverse effects were observed during the study.
Conclusion: AYUSH 64 along-with standard care in ILI is safe and efficacious and this may be used in other viral infections with pyrexia as add-on to standard care for early recovery and better outcome.
Keywords: AYUSH 64; Ayurveda intervention; Efficacy; Influenza-like illness (ILI); Safety; Vata-Kaphaja Jvara.
Copyright © 2021 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest Nil.
Figures
References
-
- Vaidya Jadavaji T.A., editor. Charaka Samhita of Acharya Charaka, Chikitsa Sthana, Jvara chikitsa, 3rd Chapter, Verse 86-87. 5th ed. Chaukambha Sanskrit Sansthan; Varanasi: 2001. p. 406.
-
- Vaidya Jadavaji T.A., editor. Charaka Samhita of Acharya Charaka, Chikitsa Sthana, Jvara chikitsa, 3rd Chapter, Verse 142-143. 5th ed. Chaukambha Sanskrit Sansthan; Varanasi: 2001. p. 409.
LinkOut - more resources
Full Text Sources