Monocyte Regulation in Homeostasis and Malignancy
- PMID: 33446416
- PMCID: PMC7877795
- DOI: 10.1016/j.it.2020.12.001
Monocyte Regulation in Homeostasis and Malignancy
Abstract
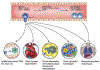
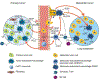
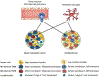
Monocytes are progenitors to macrophages and a subclass of dendritic cells (monocyte-derived dendritic cells, MoDCs), but they also act as circulating sensors that respond to environmental changes and disease. Technological advances have defined the production of classical monocytes in the bone marrow through the identification of lineage-determining transcription factors (LDTFs) and have proposed alternative routes of differentiation. Monocytes released into the circulation can be recruited to tissues by specific chemoattractants where they respond to sequential niche-specific signals that determine their differentiation into terminal effector cells. New aspects of monocyte biology in the circulation are being revealed, exemplified by the influence of cancer on the systemic alteration of monocyte subset abundance and transcriptional profiles. These changes can act to enhance the metastatic spread of primary cancers and may offer therapeutic opportunities.
Copyright © 2020 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Conflicts of Interest
JWP is a co-founder, board member and consultant to “Macomics” an immuno-oncology company. AR, CKG and CZH declare no conflicts of interest.
Figures


References
-
- Geissmann F et al. (2003) Blood Monocytes Consist of Two Principal Subsets with Distinct Migratory Properties. Immunity 19, 71–82 - PubMed
-
- Hettinger J et al. (2013) Origin of monocytes and macrophages in a committed progenitor. Nat Immunol 14, 821–830 - PubMed
-
- Fogg DK (2006) A Clonogenic Bone Marrow Progenitor Specific for Macrophages and Dendritic Cells. Science 311, 83–87 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

