A novel DNA chromatography method to discriminate Mycobacterium abscessus subspecies and macrolide susceptibility
- PMID: 33446475
- PMCID: PMC7910664
- DOI: 10.1016/j.ebiom.2020.103187
A novel DNA chromatography method to discriminate Mycobacterium abscessus subspecies and macrolide susceptibility
Abstract
Background: The clinical impact of infection with Mycobacterium (M.) abscessus complex (MABC), a group of emerging non-tuberculosis mycobacteria (NTM), is increasing. M. abscessus subsp. abscessus/bolletii frequently shows natural resistance to macrolide antibiotics, whereas M. abscessus subsp. massiliense is generally susceptible. Therefore, rapid and accurate discrimination of macrolide-susceptible MABC subgroups is required for effective clinical decisions about macrolide treatments for MABC infection. We aimed to develop a simple and rapid diagnostic that can identify MABC isolates showing macrolide susceptibility.
Methods: Whole genome sequencing (WGS) was performed for 148 clinical or environmental MABC isolates from Japan to identify genetic markers that can discriminate three MABC subspecies and the macrolide-susceptible erm(41) T28C sequevar. Using the identified genetic markers, we established PCR based- or DNA chromatography-based assays. Validation testing was performed using MABC isolates from Taiwan.
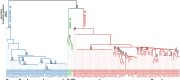
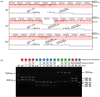
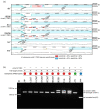
Finding: We identified unique sequence regions that could be used to differentiate the three subspecies. Our WGS-based phylogenetic analysis indicated that M. abscessus carrying the macrolide-susceptible erm(41) T28C sequevar were tightly clustered, and identified 11 genes that were significantly associated with the lineage for use as genetic markers. To detect these genetic markers and the erm(41) locus, we developed a DNA chromatography method that identified three subspecies, the erm(41) T28C sequevar and intact erm(41) for MABC in a single assay within one hour. The agreement rate between the DNA chromatography-based and WGS-based identification was 99·7%.
Interpretation: We developed a novel, rapid and simple DNA chromatography method for identification of MABC macrolide susceptibility with high accuracy.
Funding: AMED, JSPS KAKENHI.
Keywords: ATS/ERS/ESCMID/IDSA guideline; DNA chromatography; Drug susceptibility; Non-tuberculosis mycobacteria; Subspecies discrimination; Whole genome sequencing.
Copyright © 2020 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest Mitsunori Yoshida, Sotaro Sano, Shigehiko Miyamoto and Yoshihiko Hoshino are listed on a pending patent in Japan for the DNA chromatography methodology to distinguish MABC and identify macrolide susceptibility (JP2020–066277 and JP2020–066306). Dr. Morimoto and Dr Kurashima report personal fees as Consultant of INSMED, outside the submitted work. The other authors have nothing to disclose under this manuscript.
Figures


Similar articles
-
Molecular Epidemiological Characteristics of Mycobacterium abscessus Complex Derived from Non-Cystic Fibrosis Patients in Japan and Taiwan.Microbiol Spectr. 2022 Jun 29;10(3):e0057122. doi: 10.1128/spectrum.00571-22. Epub 2022 Apr 21. Microbiol Spectr. 2022. PMID: 35446117 Free PMC article.
-
Mycobacteriological characteristics and treatment outcomes in extrapulmonary Mycobacterium abscessus complex infections.Int J Infect Dis. 2017 Jul;60:49-56. doi: 10.1016/j.ijid.2017.05.007. Epub 2017 May 15. Int J Infect Dis. 2017. PMID: 28522316
-
Impact on Macrolide Resistance of Genetic Diversity of Mycobacterium abscessus Species.Microbiol Spectr. 2022 Dec 21;10(6):e0274922. doi: 10.1128/spectrum.02749-22. Epub 2022 Nov 23. Microbiol Spectr. 2022. PMID: 36416559 Free PMC article.
-
Treatment of Mycobacterium abscessus Pulmonary Disease.Chest. 2022 Jan;161(1):64-75. doi: 10.1016/j.chest.2021.07.035. Epub 2021 Jul 24. Chest. 2022. PMID: 34314673 Review.
-
Mycobacterium abscessus Complex-Associated Chronic Meningitis: Time to Think Beyond Tuberculosis.Neurol India. 2023 Sep-Oct;71(5):946-952. doi: 10.4103/0028-3886.388095. Neurol India. 2023. PMID: 37929432 Review.
Cited by
-
The Use of Comparative Genomic Analysis for the Development of Subspecies-Specific PCR Assays for Mycobacterium abscessus.Front Cell Infect Microbiol. 2022 Mar 28;12:816615. doi: 10.3389/fcimb.2022.816615. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35419298 Free PMC article.
-
Molecular Epidemiological Characteristics of Mycobacterium abscessus Complex Derived from Non-Cystic Fibrosis Patients in Japan and Taiwan.Microbiol Spectr. 2022 Jun 29;10(3):e0057122. doi: 10.1128/spectrum.00571-22. Epub 2022 Apr 21. Microbiol Spectr. 2022. PMID: 35446117 Free PMC article.
-
Diagnostic Utility of a Mycobacterium Multiplex PCR Detection Panel for Tuberculosis and Nontuberculous Mycobacterial Infections.Microbiol Spectr. 2023 Jun 15;11(3):e0516222. doi: 10.1128/spectrum.05162-22. Epub 2023 Apr 24. Microbiol Spectr. 2023. PMID: 37093012 Free PMC article.
-
Massive and Lengthy Clonal Nosocomial Expansion of Mycobacterium abscessus subsp. massiliense among Patients Who Are Ventilator Dependent without Cystic Fibrosis.Microbiol Spectr. 2023 Aug 17;11(4):e0490822. doi: 10.1128/spectrum.04908-22. Epub 2023 Jun 14. Microbiol Spectr. 2023. PMID: 37314340 Free PMC article.
-
Antibiotic-resistant status and pathogenic clonal complex of canine Streptococcus canis-associated deep pyoderma.BMC Vet Res. 2022 Nov 9;18(1):395. doi: 10.1186/s12917-022-03482-3. BMC Vet Res. 2022. PMID: 36352470 Free PMC article.
References
-
- Oren A., Garrity G.M. List of new names and new combinations previously effectively, but not validly, published. Int J Syst Evol Microbiol. 2016;66:2463–2466. - PubMed
-
- Adekambi T., Sassi M., van Ingen J., Drancourt M. Reinstating Mycobacterium massiliense and Mycobacterium bolletii as species of the Mycobacterium abscessus complex. Int J Syst Evol Microbiol. 2017;67:2726–2730. - PubMed
-
- Tortoli E., Kohl T.A., Brown-Elliott B.A., Trovato A., Cardoso-Leão S., Garcia M.J. Mycobacterium abscessus, a taxonomic puzzle. Int J Syst Evol Microbiol. 2018;68:467–469. - PubMed
-
- Griffith D.E., Aksamit T., Brown-Elliott B.A., Catanzaro A., Daley C., Gordin F. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

