An immunohistochemical study of lymphatic elements in the human brain
- PMID: 33446503
- PMCID: PMC7826383
- DOI: 10.1073/pnas.2002574118
An immunohistochemical study of lymphatic elements in the human brain
Abstract
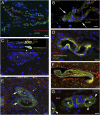
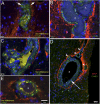
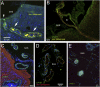
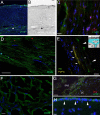
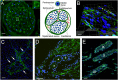
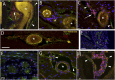
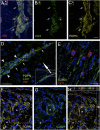
Almost 150 papers about brain lymphatics have been published in the last 150 years. Recently, the information in these papers has been synthesized into a picture of central nervous system (CNS) "glymphatics," but the fine structure of lymphatic elements in the human brain based on imaging specific markers of lymphatic endothelium has not been described. We used LYVE1 and PDPN antibodies to visualize lymphatic marker-positive cells (LMPCs) in postmortem human brain samples, meninges, cavernous sinus (cavum trigeminale), and cranial nerves and bolstered our findings with a VEGFR3 antibody. LMPCs were present in the perivascular space, the walls of small and large arteries and veins, the media of large vessels along smooth muscle cell membranes, and the vascular adventitia. Lymphatic marker staining was detected in the pia mater, in the arachnoid, in venous sinuses, and among the layers of the dura mater. There were many LMPCs in the perineurium and endoneurium of cranial nerves. Soluble waste may move from the brain parenchyma via perivascular and paravascular routes to the closest subarachnoid space and then travel along the dura mater and/or cranial nerves. Particulate waste products travel along the laminae of the dura mater toward the jugular fossa, lamina cribrosa, and perineurium of the cranial nerves to enter the cervical lymphatics. CD3-positive T cells appear to be in close proximity to LMPCs in perivascular/perineural spaces throughout the brain. Both immunostaining and qPCR confirmed the presence of adhesion molecules in the CNS known to be involved in T cell migration.
Keywords: CSF; T cells; cranial nerves; meninges; podoplanin.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Crivellato E., Travan L., Ribatti D., The Hippocratic treatise “On glands”: The first document on lymphoid tissue and lymph nodes. Leukemia 21, 591–592 (2007). - PubMed
-
- Mascagni P., Vasorum Lymphaticorum Corporis Humani Descriptio e Iconographia (Pazzini Carli, Siena, Italy, 1787).
-
- Schwalbe G., Der Arachnoidealraum, ein Lymphraum und sein Zusammenhang mit dem Perichorioidealraum. Centralblatt für die medicinischen Wissenschaften 7, 465 (1869).
-
- Key A., Retzius G., Studien in der Anatomie des Nervensystems und des Bindegewebes (Samson und Wallin, Stockholm, 1875).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

