Complement Drives Synaptic Degeneration and Progressive Cognitive Decline in the Chronic Phase after Traumatic Brain Injury
- PMID: 33446516
- PMCID: PMC8115878
- DOI: 10.1523/JNEUROSCI.1734-20.2020
Complement Drives Synaptic Degeneration and Progressive Cognitive Decline in the Chronic Phase after Traumatic Brain Injury
Abstract
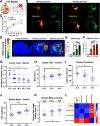
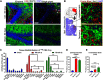
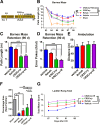
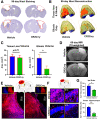
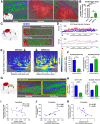
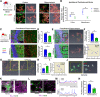
Cognitive deficits following traumatic brain injury (TBI) remain a major cause of disability and early-onset dementia, and there is increasing evidence that chronic neuroinflammation occurring after TBI plays an important role in this process. However, little is known about the molecular mechanisms responsible for triggering and maintaining chronic inflammation after TBI. Here, we identify complement, and specifically complement-mediated microglial phagocytosis of synapses, as a pathophysiological link between acute insult and a chronic neurodegenerative response that is associated with cognitive decline. Three months after an initial insult, there is ongoing complement activation in the injured brain of male C57BL/6 mice, which drives a robust chronic neuroinflammatory response extending to both hemispheres. This chronic neuroinflammatory response promotes synaptic degeneration and predicts progressive cognitive decline. Synaptic degeneration was driven by microglial phagocytosis of complement-opsonized synapses in both the ipsilateral and contralateral brain, and complement inhibition interrupted the degenerative neuroinflammatory response and reversed cognitive decline, even when therapy was delayed until 2 months after TBI. These findings provide new insight into our understanding of TBI pathology and its management; and whereas previous therapeutic investigations have focused almost exclusively on acute treatments, we show that all phases of TBI, including at chronic time points after TBI, may be amenable to therapeutic interventions, and specifically to complement inhibition.SIGNIFICANCE STATEMENT There is increasing evidence of a chronic neuroinflammatory response after traumatic brain injury (TBI), but little is known about the molecular mechanisms responsible for triggering and maintaining chronic inflammation. We identify complement, and specifically complement-mediated microglial phagocytosis of synapses, as a pathophysiological link between acute insult and a chronic neurodegenerative response, and further that this response is associated with cognitive decline. Complement inhibition interrupted this response and reversed cognitive decline, even when therapy was delayed until 2 months after injury. The data further support the concept that TBI should be considered a chronic rather than an acute disease condition, and have implications for the management of TBI in the chronic phase of injury, specifically with regard to the therapeutic application of complement inhibition.
Keywords: cognitive recovery; complement; neuroinflammation; therapy; traumatic brain injury.
Copyright © 2021 the authors.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
