An interplay of NOX1-derived ROS and oxygen determines the spermatogonial stem cell self-renewal efficiency under hypoxia
- PMID: 33446567
- PMCID: PMC7849365
- DOI: 10.1101/gad.339903.120
An interplay of NOX1-derived ROS and oxygen determines the spermatogonial stem cell self-renewal efficiency under hypoxia
Abstract
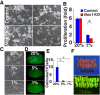
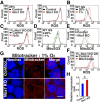
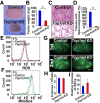
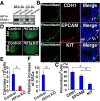
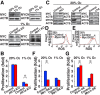
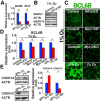
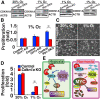
Reactive oxygen species (ROS) produced by NADPH1 oxidase 1 (NOX1) are thought to drive spermatogonial stem cell (SSC) self-renewal through feed-forward production of ROS by the ROS-BCL6B-NOX1 pathway. Here we report the critical role of oxygen on ROS-induced self-renewal. Cultured SSCs proliferated poorly and lacked BCL6B expression under hypoxia despite increase in mitochondria-derived ROS. Due to lack of ROS amplification under hypoxia, NOX1-derived ROS were significantly reduced, and Nox1-deficient SSCs proliferated poorly under hypoxia but normally under normoxia. NOX1-derived ROS also influenced hypoxic response in vivo because Nox1-deficient undifferentiated spermatogonia showed significantly reduced expression of HIF1A, a master transcription factor for hypoxic response. Hypoxia-induced poor proliferation occurred despite activation of MYC and suppression of CDKN1A by HIF1A, whose deficiency exacerbated self-renewal efficiency. Impaired proliferation of Nox1- or Hif1a-deficient SSCs under hypoxia was rescued by Cdkn1a depletion. Consistent with these observations, Cdkn1a-deficient SSCs proliferated actively only under hypoxia but not under normoxia. On the other hand, chemical suppression of mitochondria-derived ROS or Top1mt mitochondria-specific topoisomerase deficiency did not influence SSC fate, suggesting that NOX1-derived ROS play a more important role in SSCs than mitochondria-derived ROS. These results underscore the importance of ROS origin and oxygen tension on SSC self-renewal.
Keywords: Hif1; reactive oxygen species; spermatogonia.
© 2021 Morimoto et al.; Published by Cold Spring Harbor Laboratory Press.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
