Dermal fibroblasts cultured from donors with type 2 diabetes mellitus retain an epigenetic memory associated with poor wound healing responses
- PMID: 33446687
- PMCID: PMC7809350
- DOI: 10.1038/s41598-020-80072-z
Dermal fibroblasts cultured from donors with type 2 diabetes mellitus retain an epigenetic memory associated with poor wound healing responses
Abstract
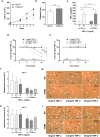
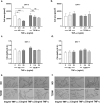
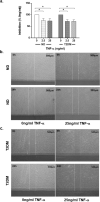
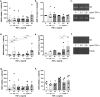
The prevalence of Type 2 diabetes mellitus (T2DM) is escalating globally. Patients suffer from multiple complications including the development of chronic wounds that can lead to amputation. These wounds are characterised by an inflammatory environment including elevated tumour necrosis factor alpha (TNF-α). Dermal fibroblasts (DF) are critical for effective wound healing, so we sought to establish whether there were any differences in DF cultured from T2DM donors or those without diabetes (ND-DF). ND- and T2DM-DF when cultured similarly in vitro secreted comparable concentrations of TNF-α. Functionally, pre-treatment with TNF-α reduced the proliferation of ND-DF and transiently altered ND-DF morphology; however, T2DM-DF were resistant to these TNF-α induced changes. In contrast, TNF-α inhibited ND- and T2DM-DF migration and matrix metalloprotease expression to the same degree, although T2DM-DF expressed significantly higher levels of tissue inhibitor of metalloproteases (TIMP)-2. Finally, TNF-α significantly increased the secretion of pro-inflammatory cytokines (including CCL2, CXCL1 and SERPINE1) in ND-DF, whilst this effect in T2DM-DF was blunted, presumably due to the tendency to higher baseline pro-inflammatory cytokine expression observed in this cell type. Collectively, these data demonstrate that T2DM-DF exhibit a selective loss of responsiveness to TNF-α, particularly regarding proliferative and secretory functions. This highlights important phenotypic changes in T2DM-DF that may explain the susceptibility to chronic wounds in these patients.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

