Formulation and in vitro evaluation of self-nanoemulsifying liquisolid tablets of furosemide
- PMID: 33446749
- PMCID: PMC7809212
- DOI: 10.1038/s41598-020-79940-5
Formulation and in vitro evaluation of self-nanoemulsifying liquisolid tablets of furosemide
Abstract
Self-nanoemulsifying drug delivery systems (SNEDDS) were used to enhance the dissolution rate of furosemide as a model for class IV drugs and the system was solidified into liquisolid tablets. SNEDDS of furosemide contained 10% Castor oil, 60% Cremophor EL, and 30% PEG 400. The mean droplets size was 17.9 ± 4.5 nm. The theoretical model was used to calculate the amounts of the carrier (Avicel PH101) and coating materials (Aerosil 200) to prepare liquisolid powder. Carrier/coating materials ratio of 5/1 was used and Ludipress was added to the solid system, thus tablets with hardness of 45 ± 2 N were obtained. Liquisolid tablets showed 2-folds increase in drug release as compared to the generic tablets after 60 min in HCl 0.1 N using USP apparatus-II. Furosemide loaded SNEDDS tablets have great prospects for further in vivo studies, and the theoretical model is useful for calculating the adequate amounts of adsorbents required to solidify these systems.
Conflict of interest statement
The authors declare no competing interests.
Figures
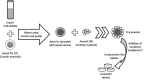


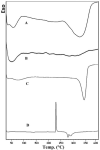
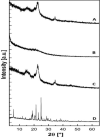


References
-
- Murtaza G, Khan SA, Najam-ul-haq M, Hussain I. Comparative evaluation of various solubility enhancement strategies for furosemide. Pak. J. Pharm. Sci. 2014;27:963–973. - PubMed
-
- Mohsin K, Pouton CW. The influence of the ratio of lipid to surfactant and the presence of cosolvent on phase behaviour during aqueous dilution of lipid-based drug delivery systems. J. Drug Deliv. Sci. Technol. 2012;22:531–540. doi: 10.1016/S1773-2247(12)50092-4. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

