Cannabis compounds exhibit anti-inflammatory activity in vitro in COVID-19-related inflammation in lung epithelial cells and pro-inflammatory activity in macrophages
- PMID: 33446817
- PMCID: PMC7809280
- DOI: 10.1038/s41598-021-81049-2
Cannabis compounds exhibit anti-inflammatory activity in vitro in COVID-19-related inflammation in lung epithelial cells and pro-inflammatory activity in macrophages
Abstract
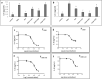
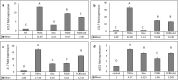
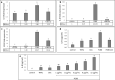
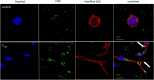
Cannabis sativa is widely used for medical purposes and has anti-inflammatory activity. This study intended to examine the anti-inflammatory activity of cannabis on immune response markers associated with coronavirus disease 2019 (COVID-19) inflammation. An extract fraction from C. sativa Arbel strain (FCBD) substantially reduced (dose dependently) interleukin (IL)-6 and -8 levels in an alveolar epithelial (A549) cell line. FCBD contained cannabidiol (CBD), cannabigerol (CBG) and tetrahydrocannabivarin (THCV), and multiple terpenes. Treatments with FCBD and a FCBD formulation using phytocannabinoid standards (FCBD:std) reduced IL-6, IL-8, C-C Motif Chemokine Ligands (CCLs) 2 and 7, and angiotensin I converting enzyme 2 (ACE2) expression in the A549 cell line. Treatment with FCBD induced macrophage (differentiated KG1 cell line) polarization and phagocytosis in vitro, and increased CD36 and type II receptor for the Fc region of IgG (FcγRII) expression. FCBD treatment also substantially increased IL-6 and IL-8 expression in macrophages. FCBD:std, while maintaining anti-inflammatory activity in alveolar epithelial cells, led to reduced phagocytosis and pro-inflammatory IL secretion in macrophages in comparison to FCBD. The phytocannabinoid formulation may show superior activity versus the cannabis-derived fraction for reduction of lung inflammation, yet there is a need of caution proposing cannabis as treatment for COVID-19.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

