In vivo multimodal optical imaging of dermoscopic equivocal melanocytic skin lesions
- PMID: 33446823
- PMCID: PMC7809210
- DOI: 10.1038/s41598-020-80744-w
In vivo multimodal optical imaging of dermoscopic equivocal melanocytic skin lesions
Abstract
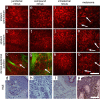
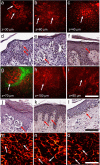
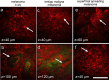
There is a wide range of equivocal melanocytic lesions that can be clinically and dermoscopically indistinguishable from early melanoma. In the present work, we assessed the possibilities of combined using of multiphoton microscopy (MPM) and optical coherence angiography (OCA) for differential diagnosis of the equivocal melanocytic lesions. Clinical and dermoscopic examinations of 60 melanocytic lesions revealed 10 benign lesions and 32 melanomas, while 18 lesions remained difficult to diagnose. Histopathological analysis of these lesions revealed 4 intradermal, 3 compound and 3 junctional nevi in the "benign" group, 7 superficial spreading, 14 lentigo maligna and 11 nodular melanomas in the "melanoma" group and 2 lentigo simplex, 4 dysplastic nevi, 6 melanomas in situ, 4 invasive lentigo melanomas and 2 invasive superficial spreading melanomas in the "equivocal" group. On the basis of MPM, a multiphoton microscopy score (MPMS) has been developed for quantitative assessment of melanoma features at the cellular level, that showed lower score for benign lesions compare with malignant ones. OCA revealed that the invasive melanoma has a higher vessel density and thicker blood vessels than melanoma in situ and benign lesions. Discriminant functions analysis of MPM and OCA data allowed to differentiate correctly between all equivocal melanocytic lesions. Therefore, we demonstrate, for the first time, that a combined use of MPM and OCA has the potential to improve early diagnosis of melanoma.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Clinical and Histopathologic Characteristics of Melanocytic Lesions on the Volar Skin Without Typical Dermoscopic Patterns.JAMA Dermatol. 2019 May 1;155(5):578-584. doi: 10.1001/jamadermatol.2018.5926. JAMA Dermatol. 2019. PMID: 30865233 Free PMC article.
-
Role of In Vivo Reflectance Confocal Microscopy in the Analysis of Melanocytic Lesions.Acta Dermatovenerol Croat. 2018 Apr;26(1):64-67. Acta Dermatovenerol Croat. 2018. PMID: 29782304 Review.
-
In vivo epiluminescence microscopy of pigmented skin lesions. II. Diagnosis of small pigmented skin lesions and early detection of malignant melanoma.J Am Acad Dermatol. 1987 Oct;17(4):584-91. doi: 10.1016/s0190-9622(87)70240-0. J Am Acad Dermatol. 1987. PMID: 3668003
-
Benign melanocytic lesions: risk markers or precursors of cutaneous melanoma?J Am Acad Dermatol. 1995 Dec;33(6):1000-7. doi: 10.1016/0190-9622(95)90294-5. J Am Acad Dermatol. 1995. PMID: 7490345
-
Dermoscopy for the pediatric dermatologist part III: dermoscopy of melanocytic lesions.Pediatr Dermatol. 2013 May-Jun;30(3):281-93. doi: 10.1111/pde.12041. Epub 2012 Dec 18. Pediatr Dermatol. 2013. PMID: 23252411 Review.
Cited by
-
Combined ultrasound and photoacoustic C-mode imaging system for skin lesion assessment.Sci Rep. 2023 Oct 20;13(1):17947. doi: 10.1038/s41598-023-44919-5. Sci Rep. 2023. PMID: 37864039 Free PMC article.
-
Non-invasive 3D imaging of human melanocytic lesions by combined ultrasound and photoacoustic tomography: a pilot study.Sci Rep. 2024 Feb 2;14(1):2768. doi: 10.1038/s41598-024-53220-y. Sci Rep. 2024. PMID: 38307985 Free PMC article.
-
Utilization of Imaging Modalities in the Diagnosis of Melanoma: A Scoping Review.Cureus. 2024 Feb 12;16(2):e54058. doi: 10.7759/cureus.54058. eCollection 2024 Feb. Cureus. 2024. PMID: 38481925 Free PMC article.
-
Multiphoton FLIM Analyses of Native and UVA-Modified Synthetic Melanins.Int J Mol Sci. 2023 Feb 24;24(5):4517. doi: 10.3390/ijms24054517. Int J Mol Sci. 2023. PMID: 36901948 Free PMC article.
-
Review of Non-Invasive Imaging Technologies for Cutaneous Melanoma.Biosensors (Basel). 2025 May 7;15(5):297. doi: 10.3390/bios15050297. Biosensors (Basel). 2025. PMID: 40422036 Free PMC article. Review.
References
-
- Anderson AM, Matsumoto M, Saul MI, Secrest AM, Ferris LK. Accuracy of skin cancer diagnosis by physician assistants compared with dermatologists in a large health care systemaccuracy of skin cancer diagnosis by physician assistants compared with dermatologistsaccuracy of skin cancer diagnosis by physician assistants compared with dermatologists. JAMA Dermatol. 2018;154:569–573. doi: 10.1001/jamadermatol.2018.0212. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

