Multimodal sensory evaluation of neuropathic spinal cord injury pain: an experimental study
- PMID: 33446934
- PMCID: PMC8338558
- DOI: 10.1038/s41393-020-00607-z
Multimodal sensory evaluation of neuropathic spinal cord injury pain: an experimental study
Abstract
Study design: An experimental study.
Objectives: To investigate the changes in somatosensory functions using the combined application of quantitative sensory testing (QST), contact heat-evoked potentials (CHEPs) and laser-evoked potentials (LEPs) studies in individuals with spinal cord injury (SCI) in relation to neuropathic pain (NeP).
Setting: Centre for Pain Medicine, Swiss Paraplegic Centre, Nottwil, Switzerland.
Methods: Individuals with SCI were compared: 12 with NeP (SCI NeP) and 12 without NeP (SCI no NeP). Tools used were QST, CHEPs, LEPs and self-reported questionnaires. Tests were applied to the control (hand) and test (dermatome of altered sensation) sites, and compared to the able-bodied group.
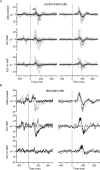
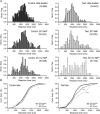
Results: QST, LEPs and CHEPs assessments showed abnormalities both on the test and control sites, which did not differ between the groups with SCI. QST showed higher prevalence of allodynia in SCI NeP. CHEPs and LEPs demonstrated diminished amplitudes in both groups with SCI in comparison to able-bodied individuals. Only reaction time (RT) analysis revealed the difference of SCI NeP from the other two groups, expressed in partially preserved responses to the laser C-fibre stimulations.
Conclusions: Combination of assessments in our study allowed to examine spinothalamic and dorsal column functions in individuals with SCI. Changes in QST, CHEPs and LEPs were detected below the level of injury independent of NeP and at the control site indicating modifications in sensory processing rostral to the spinal lesion. Analysis of RT during laser stimulation could be an essential component when evaluating the somatosensory functions related to NeP in persons with SCI.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

