SnO2-Doped ZnO/Reduced Graphene Oxide Nanocomposites: Synthesis, Characterization, and Improved Anticancer Activity via Oxidative Stress Pathway
- PMID: 33447029
- PMCID: PMC7802795
- DOI: 10.2147/IJN.S285392
SnO2-Doped ZnO/Reduced Graphene Oxide Nanocomposites: Synthesis, Characterization, and Improved Anticancer Activity via Oxidative Stress Pathway
Abstract
Background: Therapeutic selectivity and drug resistance are critical issues in cancer therapy. Currently, zinc oxide nanoparticles (ZnO NPs) hold considerable promise to tackle this problem due to their tunable physicochemical properties. This work was designed to prepare SnO2-doped ZnO NPs/reduced graphene oxide nanocomposites (SnO2-ZnO/rGO NCs) with enhanced anticancer activity and better biocompatibility than those of pure ZnO NPs.
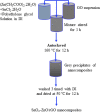
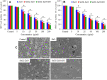
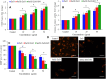
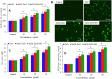
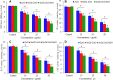
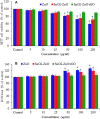
Materials and methods: Pure ZnO NPs, SnO2-doped ZnO (SnO2-ZnO) NPs, and SnO2-ZnO/rGO NCs were prepared via a facile hydrothermal method. Prepared samples were characterized by field emission transmission electron microscopy (FETEM), energy dispersive spectroscopy (EDS), field emission scanning electron microscopy (FESEM), X-ray diffraction (XRD), ultraviolet-visible (UV-VIS) spectrometer, and dynamic light scattering (DLS) techniques. Selectivity and anticancer activity of prepared samples were assessed in human breast cancer (MCF-7) and human normal breast epithelial (MCF10A) cells. Possible mechanisms of anticancer activity of prepared samples were explored through oxidative stress pathway.
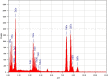
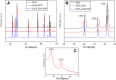
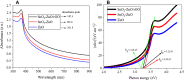
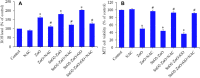
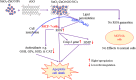
Results: XRD spectra of SnO2-ZnO/rGO NCs confirmed the formation of single-phase of hexagonal wurtzite ZnO. High resolution TEM and SEM mapping showed homogenous distribution of SnO2 and rGO in ZnO NPs with high quality lattice fringes without any distortion. Band gap energy of SnO2-ZnO/rGO NCs was lower compared to SnO2-ZnO NPs and pure ZnO NPs. The SnO2-ZnO/rGO NCs exhibited significantly higher anticancer activity against MCF-7 cancer cells than those of SnO2-ZnO NPs and ZnO NPs. The SnO2-ZnO/rGO NCs induced apoptotic response through the upregulation of caspase-3 gene and depletion of mitochondrial membrane potential. Mechanistic study indicated that SnO2-ZnO/rGO NCs kill cancer cells through oxidative stress pathway. Moreover, biocompatibility of SnO2-ZnO/rGO NCs was also higher against normal breast epithelial (MCF10A cells) in comparison to SnO2-ZnO NPs and ZnO NPs.
Conclusion: SnO2-ZnO/rGO NCs showed enhanced anticancer activity and better biocompatibility than SnO2-ZnO NPs and pure ZnO NPs. This work suggested a new approach to improve the selectivity and anticancer activity of ZnO NPs. Studies on antitumor activity of SnO2-ZnO/rGO NCs in animal models are further warranted.
Keywords: ZnO nanocomposites; better selectivity; breast cancer; caspase-3; improved anticancer activity; reactive oxygen species.
© 2021 Ahamed et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures


Similar articles
-
Facile green synthesis of ZnO-RGO nanocomposites with enhanced anticancer efficacy.Methods. 2022 Mar;199:28-36. doi: 10.1016/j.ymeth.2021.04.020. Epub 2021 Apr 27. Methods. 2022. PMID: 33930572
-
Enhanced Anticancer Performance of Eco-Friendly-Prepared Mo-ZnO/RGO Nanocomposites: Role of Oxidative Stress and Apoptosis.ACS Omega. 2022 Feb 15;7(8):7103-7115. doi: 10.1021/acsomega.1c06789. eCollection 2022 Mar 1. ACS Omega. 2022. PMID: 35252701 Free PMC article.
-
Biosynthesis, Characterization, and Augmented Anticancer Activity of ZrO2 Doped ZnO/rGO Nanocomposite.J Funct Biomater. 2023 Jan 9;14(1):38. doi: 10.3390/jfb14010038. J Funct Biomater. 2023. PMID: 36662085 Free PMC article.
-
Zinc oxide nanoparticles: Synthesis, antiseptic activity and toxicity mechanism.Adv Colloid Interface Sci. 2017 Nov;249:37-52. doi: 10.1016/j.cis.2017.07.033. Epub 2017 Aug 26. Adv Colloid Interface Sci. 2017. PMID: 28923702 Review.
-
Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications.Expert Opin Drug Deliv. 2010 Sep;7(9):1063-77. doi: 10.1517/17425247.2010.502560. Expert Opin Drug Deliv. 2010. PMID: 20716019 Free PMC article. Review.
Cited by
-
Facile Synthesis of Zn-Doped Bi2O3 Nanoparticles and Their Selective Cytotoxicity toward Cancer Cells.ACS Omega. 2021 Jun 29;6(27):17353-17361. doi: 10.1021/acsomega.1c01467. eCollection 2021 Jul 13. ACS Omega. 2021. PMID: 34278121 Free PMC article.
-
A biochemical and histology experimental approach to investigate the adverse effect of chronic lead acetate and dietary furan on rat lungs.Biometals. 2023 Feb;36(1):201-216. doi: 10.1007/s10534-022-00472-8. Epub 2022 Nov 23. Biometals. 2023. PMID: 36418810
-
Non-ROS-Mediated Cytotoxicity of ZnO and CuO in ML-1 and CA77 Thyroid Cancer Cell Lines.Int J Mol Sci. 2023 Feb 17;24(4):4055. doi: 10.3390/ijms24044055. Int J Mol Sci. 2023. PMID: 36835463 Free PMC article.
-
Casein Kinase 2 Inhibitor, CX-4945, Induces Apoptosis and Restores Blood-Brain Barrier Homeostasis in In Vitro and In Vivo Models of Glioblastoma.Cancers (Basel). 2024 Nov 24;16(23):3936. doi: 10.3390/cancers16233936. Cancers (Basel). 2024. PMID: 39682125 Free PMC article.
-
Polymeric Micelles for Breast Cancer Therapy: Recent Updates, Clinical Translation and Regulatory Considerations.Pharmaceutics. 2022 Sep 3;14(9):1860. doi: 10.3390/pharmaceutics14091860. Pharmaceutics. 2022. PMID: 36145608 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

