Molecular Biomarkers of Neovascular Age-Related Macular Degeneration With Incomplete Response to Anti-Vascular Endothelial Growth Factor Treatment
- PMID: 33447243
- PMCID: PMC7802772
- DOI: 10.3389/fphar.2020.594087
Molecular Biomarkers of Neovascular Age-Related Macular Degeneration With Incomplete Response to Anti-Vascular Endothelial Growth Factor Treatment
Abstract
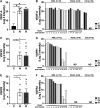
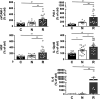
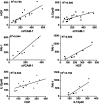
The standard treatment for neovascular age-related macular degeneration (nAMD) consists of intravitreal anti-vascular endothelial growth factors (VEGF). However, for some patients, even maximal anti-VEGF treatment does not entirely suppress exudative activity. The goal of this study was to identify molecular biomarkers in nAMD with incomplete response to anti-VEGF treatment. Aqueous humor (AH) samples were collected from three groups of patients: 17 patients with nAMD responding incompletely to anti-VEGF (18 eyes), 17 patients affected by nAMD with normal treatment response (21 eyes), and 16 control patients without any retinopathy (16 eyes). Proteomic and multiplex analyses were performed on these samples. Proteomic analyses showed that nAMD patients with incomplete anti-VEGF response displayed an increased inflammatory response, complement activation, cytolysis, protein-lipid complex, and vasculature development pathways. Multiplex analyses revealed a significant increase of soluble vascular cell adhesion molecule-1 (sVCAM-1) [ p = 0.001], interleukin-6 (IL-6) [ p = 0.009], bioactive interleukin-12 (IL-12p40) [ p = 0.03], plasminogen activator inhibitor type 1 (PAI-1) [ p = 0.004], and hepatocyte growth factor (HGF) [ p = 0.004] levels in incomplete responders in comparison to normal responders. Interestingly, the same biomarkers showed a high intercorrelation with r2 values between 0.58 and 0.94. In addition, we confirmed by AlphaLISA the increase of sVCAM-1 [ p < 0.0001] and IL-6 [ p = 0.043] in the incomplete responder group. Incomplete responders in nAMD are associated with activated angiogenic and inflammatory pathways. The residual exudative activity of nAMD despite maximal anti-VEGF treatment may be related to both angiogenic and inflammatory responses requiring specific adjuvant therapy. Data are available via ProteomeXchange with identifier PXD02247.
Keywords: Inflammation; Soluble vascular cell adhesion molecule-1 (sVCAM-1); age-related macular degeneration (AMD); angiogenic factors; bioactive interleukin-12 (IL-12p40); hepatocyte growth factor (HGF); interleukin-6 (IL-6); plasminogen ctivator inhibitor type 1 (PAI-1).
Copyright © 2020 Mantel, Borgo, Guidotti, Forestier, Kirsch, Derradji, Waridel, Burdet, Mehl, Schweizer and Roduit.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. Roy. Stat. Soc. B 57 (1), 289–300. 10.1111/j.2517-6161.1995.tb02031.x - DOI
-
- Bertelmann T., Spychalska M., Kohlberger L., Strodthoff S., Witteborn M., Kicova N., et al. (2013). Intracameral concentrations of the fibrinolytic system components in patients with age-related macular degeneration. Graefes Arch. Clin. Exp. Ophthalmol. 251 (12), 2697–2704. 10.1007/s00417-013-2374-8 - DOI - PubMed
-
- Bontzos G., Bagheri S., Ioanidi L., Kim I., Datseris I., Gragoudas E., et al. (2019). Nonresponders to ranibizumab anti-VEGF treatment are actually short-term responders: a prospective spectral-domain OCT study. Ophthalmology. Retina S2468–6530, 30628–30631. 10.1016/j.oret.2019.11.004PMID - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

