Oxidative damage, inflammation, genotoxic effect, and global DNA methylation caused by inhalation of formaldehyde and the purpose of melatonin
- PMID: 33447362
- PMCID: PMC7786178
- DOI: 10.1093/toxres/tfaa079
Oxidative damage, inflammation, genotoxic effect, and global DNA methylation caused by inhalation of formaldehyde and the purpose of melatonin
Abstract
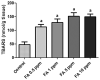
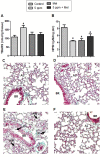
Formaldehyde (FA) exposure has been proven to increase the risk of asthma and cancer. This study aimed to evaluate for 28 days the FA inhalation effects on oxidative stress, inflammation process, genotoxicity, and global DNA methylation in mice as well as to investigate the potential protective effects of melatonin. For that, analyses were performed on lung, liver and kidney tissues, blood, and bone marrow. Bronchoalveolar lavage was used to measure inflammatory parameters. Lipid peroxidation (TBARS), protein carbonyl (PCO), non-protein thiols (NPSH), catalase activity (CAT), comet assay, micronuclei (MN), and global methylation were determined. The exposure to 5-ppm FA resulted in oxidative damage to the lung, presenting a significant increase in TBARS and NO levels and a decrease in NPSH levels, besides an increase in inflammatory cells recruited for bronchoalveolar lavage. Likewise, in the liver tissue, the exposure to 5-ppm FA increased TBARS and PCO levels and decreased NPSH levels. In addition, FA significantly induced DNA damage, evidenced by the increase of % tail moment and MN frequency. The pretreatment of mice exposed to FA applying melatonin improved inflammatory and oxidative damage in lung and liver tissues and attenuated MN formation in bone marrow cells. The pulmonary histological study reinforced the results observed in biochemical parameters, demonstrating the potential beneficial role of melatonin. Therefore, our results demonstrated that FA exposure with repeated doses might induce oxidative damage, inflammatory, and genotoxic effects, and melatonin minimized the toxic effects caused by FA inhalation in mice.
Keywords: inflammation; oxidative damage; pollutant; toxicology; xenobiotic.
© The Author(s) 2020. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures

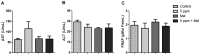
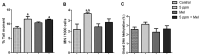
References
-
- Zendehdel R, Fazli Z, Mazinani M. Neurotoxicity effect of formaldehyde on occupational exposure and influence of individual susceptibility to some metabolism parameters. Environ Monit Assess 2016;188:648. - PubMed
-
- Zhang Q, Tian P, Zhai M et al. Formaldehyde regulates vascular tensions through nitric oxide-cGMP signaling pathway and ion channels. Chemosphere 2018;193:60–73. - PubMed
-
- Costa S, Carvalho S, Costa C et al. Increased levels of chromosomal aberrations and DNA damage in a group of workers exposed to formaldehyde. Mutagenesis 2015;30:463–73. - PubMed
-
- Barbosa E, Santos ALA, Peteffi GP et al. Increase of global DNA methylation patterns in beauty salon workers exposed to low levels of formaldehyde. Environ Sci Pollut Res 2019;26:1304–14. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

