Microvascular dysfunction in septic and dengue shock: Pathophysiology and implications for clinical management
- PMID: 33447608
- PMCID: PMC7773436
- DOI: 10.21542/gcsp.2020.29
Microvascular dysfunction in septic and dengue shock: Pathophysiology and implications for clinical management
Abstract
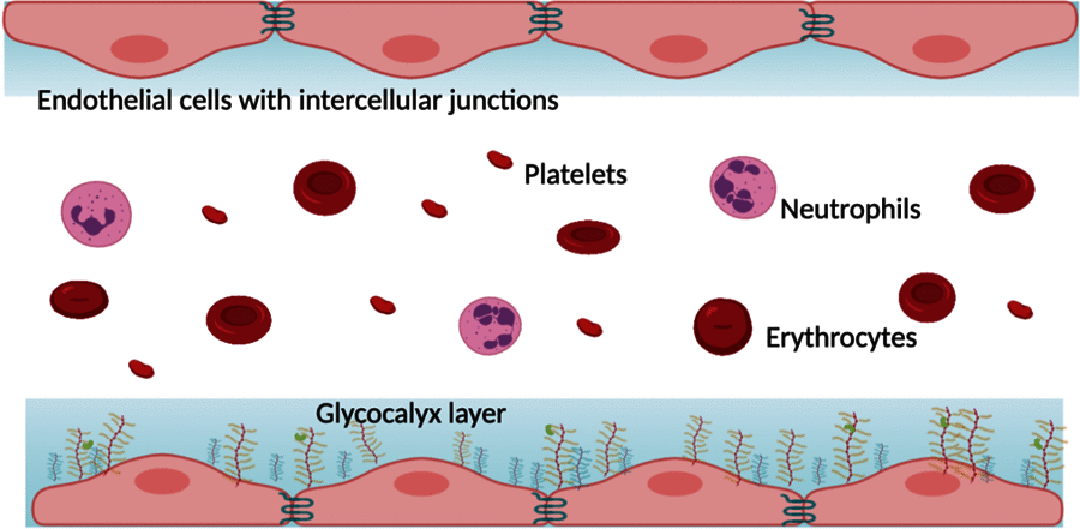
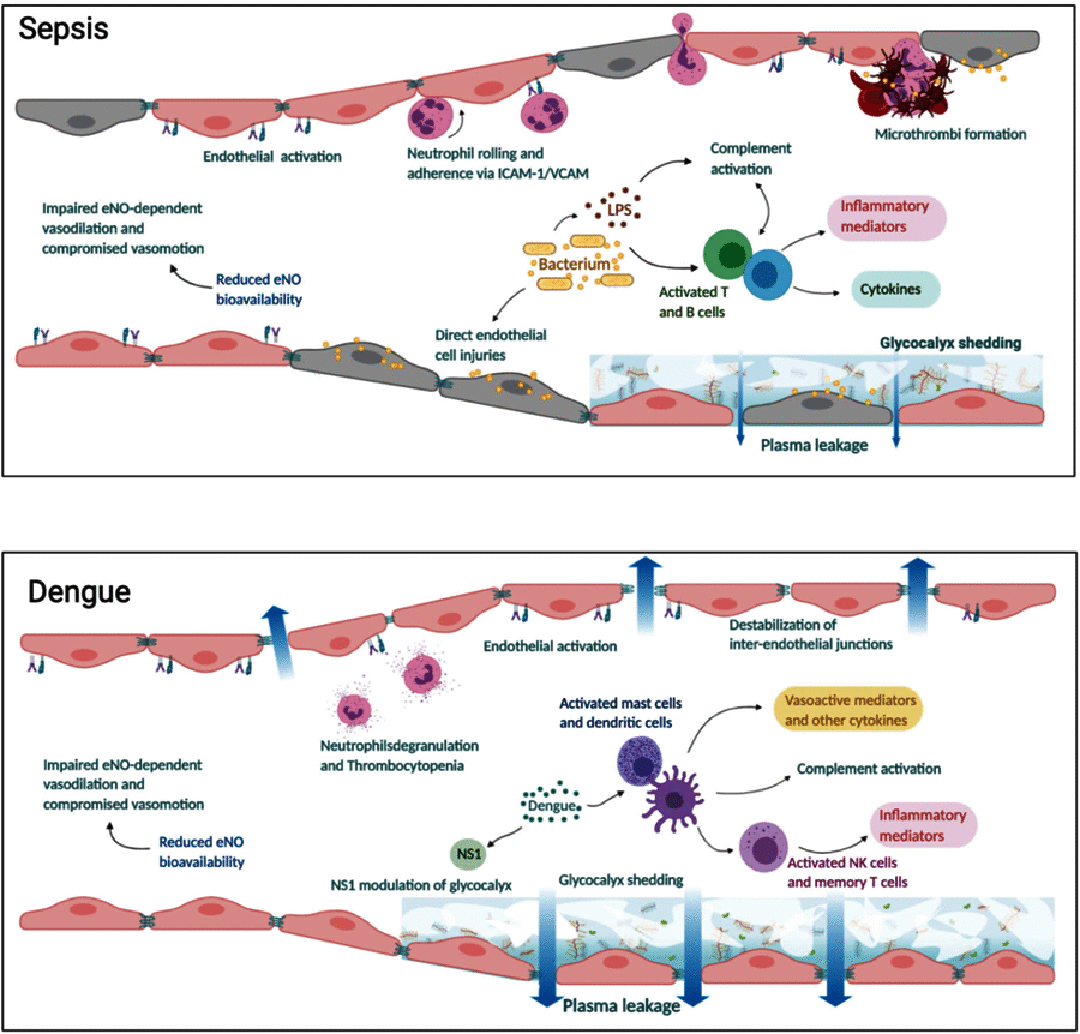
The microcirculation comprising of arterioles, capillaries and post-capillary venules is the terminal vascular network of the systemic circulation. Microvascular homeostasis, comprising of a balance between vasoconstriction, vasodilation and endothelial permeability in healthy states, regulates tissue perfusion. In severe infections, systemic inflammation occurs irrespective of the infecting microorganism(s), resulting in microcirculatory dysregulation and dysfunction, which impairs tissue perfusion and often precedes end-organ failure. The common hallmarks of microvascular dysfunction in both septic shock and dengue shock, are endothelial cell activation, glycocalyx degradation and plasma leak through a disrupted endothelial barrier. Microvascular tone is also impaired by a reduced bioavailability of nitric oxide. In vitro and in vivo studies have however demonstrated that the nature and extent of microvascular dysfunction as well as responses to volume expansion resuscitation differ in these two clinical syndromes. This review compares and contrasts the pathophysiology of microcirculatory dysfunction in septic versus dengue shock and the attendant effects of fluid administration during resuscitation.
Copyright ©2020 The Author(s).
Conflict of interest statement
SY receives consultancy fees from Janssen pharmaceuticals for dengue antiviral development and as a member of the ROCHE Advisory Board on Severe Dengue. All other authors declare no conflicts.
Figures

References
-
- Wallez Y, Huber P. Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim Biophys Acta. 2008;1778(3):794–809. - PubMed
-
- Yuan SY, Rigor RR. Regulation of endothelial barrier function. edn. San Rafael (CA); 2010. - PubMed
-
- Tarbell JM, Cancel LM. The glycocalyx and its significance in human medicine. J Intern Med. 2016;280(1):97–113. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
