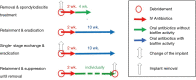
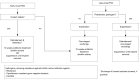
Comprehensive treatment algorithm of postoperative spinal implant infection
- PMID: 33447685
- PMCID: PMC7797808
- DOI: 10.21037/jss-20-497
Comprehensive treatment algorithm of postoperative spinal implant infection
Abstract
Postoperative spinal implant infection (PSII) is a commonly found and serious complication after instrumented spinal surgery. Whereas early-onset PSII usually can be diagnosed by clinical symptoms, the diagnosis of late-onset PSII can be often made only by examination of intraoperatively collected samples. The treatment of PSII consists of surgical and antibiotic therapy schemes. In case of early PSII, the retention of spinal implants is a feasible option, whereas late PSII is usually treated by one-staged exchange of the spinal implants. Radical debridement of surrounding tissue should be performed in any case of PSII. The antibiotic treatment depends on either the implants can be removed or need to be retained or exchanged, respectively. If the causative pathogens are sensitive for biofilm-active antibiotic agents, the duration of antibiotic treatment amounts to 12 weeks with retention of spinal implants. In case of problematic pathogens, the application of antibiotics needs to be prolonged for an individual duration. Antibiotic treatment should always be initiated with an intravenous application for at least 2 weeks.
Keywords: Revision spine surgery; infection, postoperative spinal implant infection (PSII); spine.
2020 Journal of Spine Surgery. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jss-20-497). The series “Postoperative Spinal Implant Infection” was commissioned by the editorial office without any funding or sponsorship. MP served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Journal of Spine Surgery from Nov 2018 to Nov 2020. MP also reports personal fees from Aesculap, Medtronic and SpineArt outside the submitted work. The other authors have no other conflicts of interest to declare.
Figures
References
-
- Fingar KR, Stocks C, Weiss AJ, et al. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs, Most Frequent Operating Room Procedures Performed in U.S. Hospitals, 2003-2012: Statistical Brief #186. Agency for Healthcare Research and Quality (US). Rockville (MD), 2006.
Publication types
LinkOut - more resources
Full Text Sources