Age-related changes in hippocampal-dependent synaptic plasticity and memory mediated by p75 neurotrophin receptor
- PMID: 33448137
- PMCID: PMC7884039
- DOI: 10.1111/acel.13305
Age-related changes in hippocampal-dependent synaptic plasticity and memory mediated by p75 neurotrophin receptor
Abstract
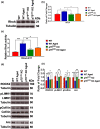
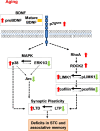
The plasticity mechanisms in the nervous system that are important for learning and memory are greatly impacted during aging. Notably, hippocampal-dependent long-term plasticity and its associative plasticity, such as synaptic tagging and capture (STC), show considerable age-related decline. The p75 neurotrophin receptor (p75NTR ) is a negative regulator of structural and functional plasticity in the brain and thus represents a potential candidate to mediate age-related alterations. However, the mechanisms by which p75NTR affects synaptic plasticity of aged neuronal networks and ultimately contribute to deficits in cognitive function have not been well characterized. Here, we report that mutant mice lacking the p75NTR were resistant to age-associated changes in long-term plasticity, associative plasticity, and associative memory. Our study shows that p75NTR is responsible for age-dependent disruption of hippocampal homeostatic plasticity by modulating several signaling pathways, including BDNF, MAPK, Arc, and RhoA-ROCK2-LIMK1-cofilin. p75NTR may thus represent an important therapeutic target for limiting the age-related memory and cognitive function deficits.
Keywords: Late-LTP; aging; hippocampus; p75NTR; synaptic capture; synaptic tagging.
© 2021 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interests.
Figures
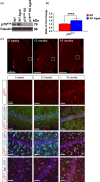



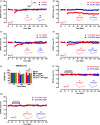

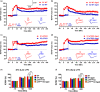


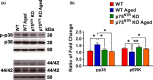
References
-
- Bach, M. E. , Barad, M. , Son, H. , Zhuo, M. , Lu, Y.‐F. , Shih, R. , Mansuy, I. , Hawkins, R. D. , & Kandel, E. R. (1999). Age‐related defects in spatial memory are correlated with defects in the late phase of hippocampal long‐term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proceedings of the National Academy of Sciences, 96, 5280–5285. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

