Dihydroartemisinin attenuates osteoarthritis by inhibiting abnormal bone remodeling and angiogenesis in subchondral bone
- PMID: 33448319
- PMCID: PMC7846423
- DOI: 10.3892/ijmm.2021.4855
Dihydroartemisinin attenuates osteoarthritis by inhibiting abnormal bone remodeling and angiogenesis in subchondral bone
Abstract
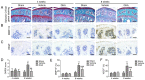
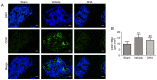
The present study aimed to investigate whether dihydroartemisinin (DHA) alleviates osteoarthritis (OA) in a mouse model of OA. Ten‑week‑old female C57BL/6j mice were used to establish OA models by anterior cruciate ligament transection (ACLT) and ovariectomized (OVX). DHA was then used to treat the OA in the ACLT and OVX mice. Safranin O‑fast green staining and Osteoarthritis Research Society International (OARSI)‑modified Mankin scores were used to grade articular cartilage degeneration. Expression of metalloproteinase‑13 (MMP‑13) and vascular endothelial growth factor (VEGF) in the articular cartilage and leukemia inhibitory factor (LIF), sclerostin, and β‑catenin in the subchondral bone were analyzed by immunohistochemistry. Expression of RANKL and CD31 were detected by immunofluorescence. Micro‑computed tomography was used to ascertain alterations in the microarchitecture of the subchondral bone. The results demonstrated that DHA decreased MMP‑13 and VEGF expression in the articular cartilage. DHA decreased OARSI scores and reduced articular cartilage degeneration. In addition, DHA reduced abnormal subchondral bone remodeling, as demonstrated by a reduction in trabecular separation (Tb.Sp), increased bone volume fractions (BV/TV), as well as bone mineral densities (BMD) compared with the ACLT+vehicle group and the OVX+vehicle group. Furthermore, DHA decreased the inhibition of sclerostin through reduction of LIF secretion by osteoclasts and, hence, attenuated aberrant bone remodeling and inhibited angiogenesis in subchondral bone, further reducing the progression of OA. The present study demonstrated that DHA attenuated OA by inhibiting abnormal bone remodeling and angiogenesis in subchondral bone, which may be a potential therapeutic target for this disease.
Keywords: osteoarthritis; subchondral bone; articular cartilage; angiogenesis; dihydroartemisinin; leukemia inhibitory factor.
Figures







References
-
- Hugle T, Geurts J. What drives osteoarthritis?-synovial versus subchondral bone pathology. Rheumatology (Oxford) 2017;56:1461–1471. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

