Inhibition of aerobic glycolysis alleviates sepsis‑induced acute kidney injury by promoting lactate/Sirtuin 3/AMPK‑regulated autophagy
- PMID: 33448325
- PMCID: PMC7849980
- DOI: 10.3892/ijmm.2021.4852
Inhibition of aerobic glycolysis alleviates sepsis‑induced acute kidney injury by promoting lactate/Sirtuin 3/AMPK‑regulated autophagy
Abstract
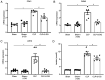
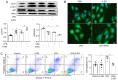
Metabolism reprogramming influences the severity of organ dysfunction, progression to fibrosis, and development of disease in acute kidney injury (AKI). Previously we showed that inhibition of aerobic glycolysis improved survival rates and protected septic mice from kidney injury. However, the underlying mechanisms remain unclear. In the present study, it was revealed that sepsis or lipopolysaccharide (LPS) enhanced aerobic glycolysis as evidenced by increased lactate production and upregulated mRNA expression of glycolysis‑related genes in kidney tissues and human renal tubular epithelial (HK‑2) cells. The aerobic glycolysis inhibitor 2‑deoxy‑D‑glucose (2‑DG) downregulated glycolysis, and improved kidney injury induced by sepsis. 2‑DG treatments increased the expression of sirtuin 3 (SIRT3) and phosphorylation‑AMP‑activated protein kinase (p‑AMPK), following promoted autophagy and attenuated apoptosis of tubular epithelial cells in septic mice and in LPS‑treated HK‑2 cells. However, the glycolysis metabolite lactate downregulated SIRT3 and p‑AMPK expression, inhibited autophagy and enhanced apoptosis in LPS‑treated HK‑2 cells. Furthermore, pharmacological blockade of autophagy with 3‑methyladenine (3‑MA) partially abolished the protective effect of 2‑DG in sepsis‑induced AKI. These findings indicated that inhibition of aerobic glycolysis protected against sepsis‑induced AKI by promoting autophagy via the lactate/SIRT3/AMPK pathway.
Keywords: aerobic glycolysis; 2‑deoxy‑D‑glucose; autophagy; acute kidney injury; sepsis.
Figures






References
-
- Cruz MG, Dantas JG, Levi TM, Rocha Mde S, de Souza SP, Boa-Sorte N, de Moura CG, Cruz CM. Septic versus non-septic acute kidney injury in critically ill patients: Characteristics and clinical outcomes. Rev Bras Ter Intensiva. 2014;26:384–391. doi: 10.5935/0103-507X.20140059. In En, Portuguese. - DOI - PMC - PubMed
-
- Mehta RL, Bouchard J, Soroko SB, Ikizler TA, Paganini EP, Chertow GM, Himmelfarb J, Program to Improve Care in Acute Renal Disease (PICARD) Study Group Sepsis as a cause and consequence of acute kidney injury: Program to improve care in acute renal disease. Intensive Care Med. 2011;37:241–248. doi: 10.1007/s00134-010-2089-9. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

