Prophylactic antibiotics for adults with chronic obstructive pulmonary disease: a network meta-analysis
- PMID: 33448349
- PMCID: PMC8092479
- DOI: 10.1002/14651858.CD013198.pub2
Prophylactic antibiotics for adults with chronic obstructive pulmonary disease: a network meta-analysis
Abstract
Background: Chronic obstructive pulmonary disease (COPD) is a chronic respiratory condition characterised by persistent respiratory symptoms and airflow limitation. Acute exacerbations punctuate the natural history of COPD and are associated with increased morbidity and mortality and disease progression. Chronic airflow limitation is caused by a combination of small airways (bronchitis) and parenchymal destruction (emphysema), which can impact day-to-day activities and overall quality of life. In carefully selected patients with COPD, long-term, prophylactic use of antibiotics may reduce bacterial load, inflammation of the airways, and the frequency of exacerbations.
Objectives: To assess effects of different prophylactic antibiotics on exacerbations, quality of life, and serious adverse events in people with COPD in three separate network meta-analyses (NMAs), and to provide rankings of identified antibiotics.
Search methods: To identify eligible randomised controlled trials (RCTs), we searched the Cochrane Airways Group Specialised Register of trials and clinical trials registries. We conducted the most recent search on 22 January 2020.
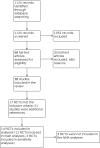
Selection criteria: We included RCTs with a parallel design of at least 12 weeks' duration evaluating long-term administration of antibiotics prophylactically compared with other antibiotics, or placebo, for patients with COPD.
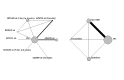
Data collection and analysis: This Cochrane Review collected and updated pair-wise data from two previous Cochrane Reviews. Searches were updated and additional studies included. We conducted three separate network meta-analyses (NMAs) within a Bayesian framework to assess three outcomes: exacerbations, quality of life, and serious adverse events. For quality of life, we collected data from St George's Respiratory Questionnaire (SGRQ). Using previously validated methods, we selected the simplest model that could adequately fit the data for every analysis. We used threshold analysis to indicate which results were robust to potential biases, taking into account each study's contributions to the overall results and network structure. Probability ranking was performed for each antibiotic class for exacerbations, quality of life, and serious adverse events.
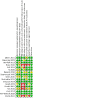
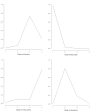
Main results: Characteristics of studies and participants Eight trials were conducted at multiple sites that included hospital clinics or academic health centres. Seven were single-centre trials conducted in hospital clinics. Two trials did not report settings. Trials durations ranged from 12 to 52 weeks. Most participants had moderate to severe disease. Mean age ranged from 64 years to 73 years, and more males were recruited (51% to 100%). Forced expiratory volume in one second (FEV₁) ranged from 0.935 to 1.36 L. Most participants had previous exacerbations. Data from 12 studies were included in the NMAs (3405 participants; 16 treatment arms including placebo). Prophylactic antibiotics evaluated were macrolides (azithromycin and erythromycin), tetracyclines (doxycyclines), quinolones (moxifloxacin) and macrolides plus tetracyclines (roxithromycin plus doxycycline). Risk of bias and threshold analysis Most studies were at low risk across domains, except detection bias, for which only seven studies were judged at low risk. In the threshold analysis for exacerbations, all comparisons in which one antibiotic was compared with another were robust to sampling variation, especially macrolide comparisons. Comparisons of classes with placebo were sensitive to potential bias, especially macrolide versus placebo, therefore, any bias in the comparison was likely to favour the active class, so any adjustment would bring the estimated relative effect closer to the null value, thus quinolone may become the best class to prevent exacerbations. Exacerbations Nine studies were included (2732 participants) in this NMA (exacerbations analysed as time to first exacerbation or people with one or more exacerbations). Macrolides and quinolones reduced exacerbations. Macrolides had a greater effect in reducing exacerbations compared with placebo (macrolides: hazard ratio (HR) 0.67, 95% credible interval (CrI) 0.60 to 0.75; quinolones: HR 0.89, 95% CrI 0.75 to 1.04), resulting in 127 fewer people per 1000 experiencing exacerbations on macrolides. The difference in exacerbations between tetracyclines and placebo was uncertain (HR 1.29, 95% CrI 0.66 to 2.41). Macrolides ranked first (95% CrI first to second), with quinolones ranked second (95% CrI second to third). Tetracyclines ranked fourth, which was lower than placebo (ranked third). Contributing studies were considered as low risk of bias in a threshold analysis. Quality of life (SGRQ) Seven studies were included (2237 participants) in this NMA. SGRQ scores improved with macrolide treatment compared with placebo (fixed effect-fixed class effect: mean difference (MD) -2.30, 95% CrI -3.61 to -0.99), but the mean difference did not reach the minimally clinical important difference (MCID) of 4 points. Tetracyclines and quinolones did not improve quality of life any more than placebo, and we did not detect a difference between antibiotic classes. Serious adverse events Nine studies were included (3180 participants) in the NMA. Macrolides reduced the odds of a serious adverse event compared with placebo (fixed effect-fixed class effect: odds ratio (OR) 0.76, 95% CrI 0.62 to 0.93). There was probably little to no difference in the effect of quinolone compared with placebo or tetracycline plus macrolide compared with placebo. There was probably little to no difference in serious adverse events between quinolones or tetracycline plus macrolide. With macrolide treatment 49 fewer people per 1000 experienced a serious adverse event compared with those given placebo. Macrolides ranked first, followed by quinolones. Tetracycline did not rank better than placebo. Drug resistance Ten studies reported drug resistance. Results were not combined due to variation in outcome measures. All studies concluded that prophylactic antibiotic administration was associated with the development of antimicrobial resistance.
Authors' conclusions: This NMA evaluated the safety and efficacy of different antibiotics used prophylactically for COPD patients. Compared to placebo, prolonged administration of macrolides (ranked first) appeared beneficial in prolonging the time to next exacerbation, improving quality of life, and reducing serious adverse events. No clear benefits were associated with use of quinolones or tetracyclines. In addition, antibiotic resistance was a concern and could not be thoroughly assessed in this review. Given the trade-off between effectiveness, safety, and risk of antibiotic resistance, prophylactic administration of antibiotics may be best reserved for selected patients, such as those experiencing frequent exacerbations. However, none of the eligible studies excluded patients with previously isolated non-tuberculous mycobacteria, which would contraindicate prophylactic administration of antibiotics, due to the risk of developing resistant non-tuberculous mycobacteria.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
SJ is employed full‐time by an NIHR Programme Grant to complete work on this Cochrane Review.
SD was a co‐applicant on a grant whereby Pfizer partially sponsored a researcher but was not sponsored by Pfizer herself.
CT was employed part‐time in 2017‐18 by an NIHR Programme Grant to complete work on this Cochrane Review. He is currently a Specialty Registrar in Clinical Pharmacology and Therapeutics and General Internal Medicine.
AGM has received a research grant for an investigator‐initiated study by Boehringer Ingelheim. He has received honoraria from Boehringer Ingelheim and GlaxoSmithKline, which are not related to the content of this manuscript.
RF is employed part‐time by an NIHR Programme Grant to complete work on this Cochrane Review and is a qualified general practitioner.
RW is a research fellow employed by the University of York.
SS is a research fellow employed by the University of York.
Figures






Comment in
-
In COPD, prophylactic macrolides, but not tetracyclines or quinolones, reduce exacerbations, with fewer serious adverse events.Ann Intern Med. 2021 Jun;174(6):JC66. doi: 10.7326/ACPJ202106150-066. Epub 2021 Jun 1. Ann Intern Med. 2021. PMID: 34058115
References
References to studies included in this review
Albert 2011 {published data only}
-
- Albert R, Bailey WC, Casaburi R, Connett J, Cooper A, Criner GJ, et al. Chronic azithromycin decreases the frequency of chronic obstructive pulmonary disease exacerbations. American Journal of Respiratory and Critical Care Medicine 2011;183:A6416.
-
- Albert R, Connett J, Bailey WC, Casaburi R, Cooper JAD, Criner GJ, et al. Long-term azithromycin decreases COPD exacerbations. Respirology 2013;18:5.
-
- Correction: Azithromycin for prevention of exacerbations of COPD. New England Journal of Medicine 2012;366:14.
Banerjee 2005 {published data only}
-
- Banerjee D, Honeybourne D, Khair OA. The effect of oral clarithromycin on bronchial airway inflammation in moderate-to-severe stable COPD: a randomised controlled trial. Treatments in Respiratory Medicine 2004;3:59-65. - PubMed
Berkhof 2013 {published data only}
-
- Berkhof FF, Ten Hertog NE, Uil SM, Kerstjens HAM, Van Den Berg JK, CACTUS Study Group. Randomized controlled trial of prophylactic azithromycin on cough-specific health status in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2013;187:A2449. - PMC - PubMed
Blasi 2010 {published data only}
Brill 2015 {published data only}
-
- Brill S, James P, Cuthbertson L, Cookson W, Moffatt M, Wedzicha J. Haemophilus dominance of the stable COPD microbiome is associated with greater bacterial load and inflammation and is modulated by prophylactic antibiotic therapy. European Respiratory Journal 2015;46:OA4746.
-
- Brill S, Law M, Allinson J, El-Emir E, McHugh T, Donaldson G, et al. Bacterial resistance induction with prophylactic antibiotics in COPD. In: European Respiratory Journal. Vol. 44. 2014:P4731.
-
- Brill SE, Law M, El-Emir E, Allinson P, Nazareth I, Donaldson GC, et al. Effect of antibiotics on airway bacteria in patients with chronic obstructive pulmonary disease. In: American Journal of Respiratory and Critical Care Medicine. Vol. 198. 2014:A2874.
He 2010 {published data only}
Mygind 2010 {published data only}
-
- Mygind LH, Pedersen C, Vestbo J, Christensen JJ, Frimodt-Moller N, Kristiansen IS, et al. A randomised, placebo-controlled 3 years study of prophylactic azithromycin in 575 patients with chronic obstructive pulmonary disease. In: European Respiratory Society 20th Annual Congress; 2010 Sep 18-22; Barcelona. 2010.
Seemungal 2008 {published data only}
-
- Seemungal TA, Wilkinson TM, Hurst JR, Perera WR, Sapsford RJ, Wedzicha JA. Long-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations. American Journal of Respiratory and Critical Care Medicine 2008;178:1139-47. - PubMed
Sethi 2010 {published data only}
Shafuddin 2015 {published data only}
-
- Shafuddin E, Mills GD, Holmes MD, Poole PJ, Mullins PR, Black PN. A double-blind, randomised, placebo-controlled study of roxithromycin and doxycycline combination, roxithromycin alone, or matching placebo for 12 weeks in adults with frequent exacerbations of chronic obstructive pulmonary disease. Journal of Negative Results in Biomedicine 2015;14:15. [DOI: 10.1186/s12952-015-0034-8] - DOI - PMC - PubMed
Simpson 2014 {published data only}
Singh 2019 {published data only}
Suzuki 2001 {published data only}
-
- Suzuki T, Yani M, Yamaya M, Satoh Nakagawa T, Sekizawa K, Ishida S, et al. Erythromycin and common cold in COPD. Chest 2001;120:730-3. - PubMed
Tan 2016 {published data only}
-
- Tan C , Huang H , Zhang J , He Z , Zhong X, Bai J. Effects of low-dose and long-term treatment with erythromycin on interleukin-17 and interleukin-23 in peripheral blood and induced sputum in patients with stable chronic obstructive pulmonary disease. Mediators of Inflammation 2016;4173962:1-11. [DOI: 10.1155/2016/4173962] - DOI - PMC - PubMed
Uzun 2014 {published data only}
-
- Djamin RS, Uzun S, Ermens AAM, Kerstens R, Hoogsteden HC, Aerts JGJV, et al. Which predictors in COPD patients with the frequent exacerbator phenotype predict the treatment response to maintenance therapy with azithromycin? In: European Respiratory Journal. Vol. 48. 2016:PA3713.
-
- Uzun S, Djamin RS, Aerts JGJV, Van Der Eerden MM. Patients with COPD Gold C & D: the effect of long-term treatment with azithromycin on exacerbation risk assessed by the Gold Framework. In: American Journal of Respiratory and Critical Care Medicine. Vol. 189. 2014:A5967.
-
- Uzun S, Djamin RS, Kluytmans JA, Mulder PG, Van't Veer NE, Ermens AA, et al. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomised, double-blind,placebo-controlled trial. Lancet Respiratory Medicine 2014;2(5):361-8. - PubMed
-
- Uzun S, Djamin RS, Mulder PGH, Kluytmans JAJW, Pelle AJ, Van't Veer NE, et al. Effect of azithromycin maintenance treatment in patients with frequent exacerbations of COPD (COLUMBUS): a randomised, double-blind, placebo-controlled trial. In: American Journal of Respiratory and Critical Care Medicine. Vol. 189. 2014:A2884. - PubMed
Vermeersch 2019 {published data only}
-
- Vermeersch K, Gabrovska M, Aumann J, Demedts IK, Corhay J-L, Marchard E, et al. Azithromycin during acute chronic obstructive pulmonary disease exacerbations requiring hospitalisation (BACE). A multi-centre, randomised, double-blind, placebo-controlled trial. American Journal of Respiratory and Critical Care Medicine 2019;200(7):857-68. [10.1164/rccm.201901-0094oc] - PubMed
References to studies excluded from this review
Bussi 1980 {published data only}
-
- Bussi S, Murciano D, Botto MJ, Pariente R. Assessment of chemoprophylaxis with intermittent tetracycline in chronic bronchitis. A functional follow-up for 3 years. Revue Francaise des Malades Respiratoires 1980;8(5):351-6. - PubMed
Calder 1968 {published data only}
-
- Calder MA, Lutz W, Schonell ME. A five year study of bacteriology and prophylactic chemotherapy in patients with chronic bronchitis. British Journal of Diseases of the Chest 1968;62:93. - PubMed
Cooper 1961 {published data only}
-
- Cooper AW, Williamson GM, Zinnemann K. Chronic bronchitis: changes in the bacterial flora of the sputum associated with exacerbations and long-term antibacterial treatment. British Journal of Diseases of the Chest 1961;55:23. - PubMed
Edwards 1958 {published data only}
EUCTR2011‐004351‐39‐IT {published data only}
-
- EUCTR2011-004351-39-IT. Phase II, randomised, double arm, multi-centre study evaluating the efficacy and safety of azithromycin for the long term prophylactic treatment of COPD in primary antibody deficiency patients with clinical spirometrically confirmed COPD suffering from repeated acute exacerbations. www.clinicaltrialsregister.eu/ctr-search/search?query=2011-004351-39 (first received 28 October 2011).
Francis 1960 {published data only}
ISRCTN72035428 {published data only}
-
- ISRCTN72035428. The effectiveness of antibiotics compared to no antibiotics for exacerbations of chronic obstructive pulmonary disease: a feasibility study. www.isrctn.com/ISRCTN72035428 (first received 12 March 2010).
Milito 2019 {published data only}
-
- Milito C, Pulvirenti F, Tabolli S, Carello R, Quinti I. Antibiotic prophylaxis in primary antibody deficiency patients: study design. Journal of Clinical Immunology 2017;37:197-266.
-
- Milito C, Pulvirenti F, Tabolli S, Carrabba M, Fabio G, Rizzo F, et al. Low dose azithromycin prophylaxis in primary antibody deficiencies. European Respiratory Journal 2019;54(Suppl 63):RCT5100. [DOI: 10.1183/13993003.congress-2019.RCT5100] - DOI
Murdoch 1959 {published data only}
Nicholson 2016 {published data only}
-
- Nicholson TT, Franciosi A, Landers S, Butler MW. Assessing potential risks of treatment with long-term azithromycin in COPD patients: long-term oxygen users beware? Irish Journal of Medical Science 2016;185(4):993-7. - PubMed
O'Reilly 2013 {published data only}
Velzen 2016 {published data only}
-
- Van Velzen P, Ter Riet G, Bresser P, Van Den Berg BTJ, Van Den Berg JWK, Daniels JMA, et al. Long term effects of antibiotics in COPD exacerbations: a randomised clinical trial. American Journal of Respiratory and Critical Care Medicine 2016;193:A1021.
Watanabe 1994 {published data only}
-
- Watanabe M, Motomiya T, Nuwika Y, Nakai Y, Honda K, Konno K, et al. A well-controlled comparative clinical study of the combination regimen of ciprofloxacin plus erythromycin for the treatment of repeated acute exacerbations of chronic respiratory tract infections. Chemotherapy 1994;42(10):1194-201.
Zykov 2008 {published data only}
-
- Zykov K, Rvatcheva A, Pustovalov A, Averyanov A. Long term treatment with clarithromycin of stage II COPD patients with frequent exacerbations. In: European Respiratory Society 18th Annual Congress; 2008 Oct 3-7; Berlin. 2008.
Additional references
Brusselle 2013
-
- Brusselle GG, Vanderstichele C, Jordens P, Deman R, Slabbynck H, Ringoet V, et al. Azithromycin for prevention of exacerbations in severe asthma (AZIAST): a multi-centre randomised double-blind placebo-controlled trial. Thorax 2013;68(4):322-9. - PubMed
Cui 2018
Dias 2013a
Dias 2013b
Dias 2018
-
- Dias S, Ades AE, Welton NJ, Jansen JP, Sutton AJ. Bias adjustment methods. In: Dias S, Ades AE, Welton NJ, Jansen JP, Sutton AJ, editor(s). In: Network Meta-Analysis for Decision-Making. New York: John Wiley and Sons Ltd., 2018. [DOI: 10.1002/9781118951651.ch9] - DOI
Donaldson 2002
Donath 2013
-
- Donath E, Chaudhry A, Hernandez-Aya LF, Lit L. A meta-analysis on the prophylactic use of macrolide antibiotics for the prevention of disease exacerbations in patients with chronic obstructive pulmonary disease. Respiratory Medicine 2013;107:1385-92. - PubMed
Franchini 2012
-
- Franchini A, Dias S, Ades AE, Jansen J, Welton N. Accounting for correlation in mixed treatment comparisons with multi-arm trials. Research Synthesis Methods 2012;3:142-60. - PubMed
GOLD 2020
-
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2020 Report. www.guidelines.co.uk/respiratory/gold-copd-2020-strategy/455088.article (accessed 1 May 2020).
Guyatt 2017
-
- Guyatt GH, Ebrahim S, Alonso-Coello P, Johnston BC, Mathioudakis AG, Briel M, et al. GRADE Guidelines 17: Assessing the risk of bias associated with missing participant outcome data in a body of evidence. Journal of Clinical Epidemiology 2017;87:14-22. - PubMed
Herath 2018
Higgins 2018
-
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Huckle 2018
-
- Huckle AW, Fairlough LC, Todd IT. Prophylactic antibiotic use in COPD and the potential anti-inflammatory activities of antibiotics. Respiratory Care 2018;63(5):609-19. - PubMed
Hurst 2010
Janjua 2021
Kew 2014
Lee 2013
-
- Lee JS, Park D-A, Hong Y, Jo KW, Lee SW, Huh JW, et al. Systematic review and meta-analysis of prophylactic antibiotics in COPD and/or chronic bronchitis. International Journal of Tuberculosis and Lung Disease 2013;17(2):153-62. - PubMed
Loukides 2013
-
- Loukides S, Bartziokas K, Vestbo J, Singh D. Novel anti-inflammatory agents in COPD: targeting lung and systemic inflammation. Current Drug Targets 2013;14(2):235-45. - PubMed
Lunn 2009
-
- Lunn D, Spiegelhalter D, Thomas A, Best N. The BUGS project: evolution, critique and future directions (with discussion). Statistics In Medicine 2009;28:3049-82. - PubMed
Martinez 2008
Mathioudakis 2017
Mathioudakis 2020
Miravittles 2015
Moher 2009
NCT00524095
-
- NCT00524095. Bronchiectasis in Chronic Obstructive Pulmonary Disease (COPD) Patients: Role of Prophylaxis. https://clinicaltrials.gov/ct2/show/NCT00524095 2009.
NCT02628769
-
- NCT02628769. A study to evaluate the anti-inflammatory effects of solithromycin in chronic obstructive pulmonary disease. https://clinicaltrials.gov/ct2/show/NCT02628769 2017.
Ni 2015
NICE 2016
-
- National Institute of Health and Clinical Excellence. Chronic obstructive pulmonary disease in over 16s: diagnosis and management (NG115). www.nice.org.uk/guidance/ng115 (accessed prior to 18 August 2020).
NICE 2018
-
- National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease in 16s: diagnosis and management NG115. Recommendations. www.nice.org.uk/guidance/ng115/chapter/Recommendations (accessed prior to 18 August 2020).
Pasquale 2012
-
- Pasquale MK, Sun SX, Song F, Hartnett HJ, Stemkowski SA. Impact of exacerbations on health care cost and resource utilization in chronic obstructive pulmonary disease patients with chronic bronchitis from a predominantly Medicare population. International Journal of Chronic Obstructive Pulmonary Disease 2012;7:757-64. [DOI: 10.2147/COPD.S36997] - DOI - PMC - PubMed
Phillippo 2018
Phillippo 2019
-
- Phillippo DM, Dias S, Welton NJ, Caldwell DM, Taske N, Ades AE. Threshold analysis as an alternative to GRADE for assessing confidence in guideline recommendations based on network meta-analyses threshold analysis in guideline development. Annals of Internal Medicine 2019;170(8):538-46. - PMC - PubMed
Puhan 2006
Rhodes 2015
Rubinstein 2002
-
- Rubinstein I, Camm J. Cardiotoxicity of fluoroquinolones. Journal of Antimicrobial Chemotherapy 2002;49(4):593-6. - PubMed
Sapey 2006
Seemungal 1998
-
- Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Americal Journal of Respiratory and Critical Care Medicine 1998;157(5 Pt 1):1418-22. - PubMed
Sethi 2004
-
- Sethi S. Bacteria in exacerbations of chronic obstructive pulmonary disease: phenomenon or epiphenomenon? Proceedings of the American Thoracic Society 2004;1(2):109-14. - PubMed
Siva 2014
Smith 2020
-
- Smith D, Du Rand I, Addy CL, Collyns T, Hart SP, Mitchelmore PJ, et al. British Thoracic Society guideline for the use of long-term macrolides in adults with respiratory disease. British Medical Journal 2020;0:1-35. [DOI: 10.1136/ thoraxjnl-2019-213929] - PubMed
Soler‐Cataluña 2005
Sze 2014
Turner 2015
Wang 2018
-
- Wang Y, Zijp TR, Bahar MA, Kocks JWH, Wilffert B, Hak E. Effects of prophylactic antibiotics on patients with stable COPD: a systematic review and meta-analysis of randomised controlled trials. Journal of Antimicrobial Chemotherapy 2018;73:3231-43. - PubMed
Yao 2013
-
- Yao G-Y, Ma Y-L, Zhang M-O, Gao Z-C. Macrolide therapy decreases chronic obstructive pulmonary disease exacerbation: a meta-analysis. Respiration 2013;86:254-60. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

