Monitoring Adherence Rate to Growth Hormone Therapy and Growth Outcomes in Taiwanese Children Using Easypod Connect: Observational Study
- PMID: 33448936
- PMCID: PMC7846437
- DOI: 10.2196/14774
Monitoring Adherence Rate to Growth Hormone Therapy and Growth Outcomes in Taiwanese Children Using Easypod Connect: Observational Study
Abstract
Background: Adherence to growth hormone therapy is difficult to detect reliably. Devices such as easypod have been developed for electronic recording of injections. The easypod connect observational study (ECOS) was an open-label, observational, multinational, phase IV study conducted in 24 countries around the world. The final results from ECOS in the Taiwanese cohort are reported in this paper.
Objective: This study aimed to evaluate the adherence and long-term outcomes of growth hormone therapy in pediatric subjects using the easypod electromechanical device.
Methods: Subjects (aged 2-18 years or >18 years without fusion of growth plates) who received Saizen (recombinant human growth hormone, somatropin) via the easypod device were enrolled in this study. The primary objective was to assess the level of adherence in subjects receiving Saizen via easypod.
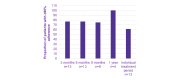
Results: In Taiwan, a total of 35 and 13 children fulfilled the criteria of full analysis set and complete analysis set, respectively. The mean (SD) age of the complete analysis set was 12.08 (2.72) years. All subjects were growth hormone-naïve, with 38% (5/13) females. The mean adherence rates of 13 subjects were 87.6% at 3 months and 84.3% at 6 months, that of 8 subjects was 81.0% at 9 months, and that of 4 subjects was 91.6% at 1 year. After 1 year of treatment, subjects had a median (Q1:Q3) change in height SD score of 0.30 (0.06:0.48), median height velocity of 6.50 (4.33:8.24) cm/year, and median change in height velocity SD score of 1.81 (-0.04:3.52).
Conclusions: With the easypod device, patients with inadequate adherence and poor response to treatment can be identified. Adherence to growth hormone therapy administered via easypod was generally high in the first year of treatment but the adherence gradually decreased over time. Overall, growth outcomes after 1 year indicated a positive growth response to growth hormone treatment. Future efforts should be focused on personalized management of adherence by using the easypod system.
Keywords: adherence; eHealth; easypod; growth hormone.
©Pen-Hua Su, Chen Yang, Mei-Chyn Chao, Chung-Lin Chiang. Originally published in JMIR Pediatrics and Parenting (http://pediatrics.jmir.org), 15.01.2021.
Conflict of interest statement
Conflicts of Interest: PHS, CY, and MCC declare no conflicts of interest. CLC is an employee of Merck Ltd.
Figures


References
-
- Cappa M, Iughetti L, Loche S, Maghnie M, Vottero A, GeNeSIS National Board on behalf of the GeNeSIS Italian Investigators Efficacy and safety of growth hormone treatment in children with short stature: the Italian cohort of the GeNeSIS clinical study. J Endocrinol Invest. 2016 Jun;39(6):667–77. doi: 10.1007/s40618-015-0418-0. http://europepmc.org/abstract/MED/27223400 - DOI - PMC - PubMed
-
- Tanaka T, Yokoya S, Hoshino Y, Hiro S, Ohki N. Long-term safety and efficacy of daily recombinant human growth hormone treatment in Japanese short children born small for gestational age: final report from an open and multi-center study. Clin Pediatr Endocrinol. 2018;27(3):145–157. doi: 10.1297/cpe.27.145. http://europepmc.org/abstract/MED/30083031 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

