Mechanisms of meiotic drive in symmetric and asymmetric meiosis
- PMID: 33449147
- PMCID: PMC8043981
- DOI: 10.1007/s00018-020-03735-0
Mechanisms of meiotic drive in symmetric and asymmetric meiosis
Abstract
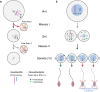
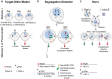
Meiotic drive, the non-Mendelian transmission of chromosomes to the next generation, functions in asymmetric or symmetric meiosis across unicellular and multicellular organisms. In asymmetric meiosis, meiotic drivers act to alter a chromosome's spatial position in a single egg. In symmetric meiosis, meiotic drivers cause phenotypic differences between gametes with and without the driver. Here we discuss existing models of meiotic drive, highlighting the underlying mechanisms and regulation governing systems for which the most is known. We focus on outstanding questions surrounding these examples and speculate on how new meiotic drive systems evolve and how to detect them.
Keywords: Germ cells; Meiosis; Meiotic drive; Selfish genetic elements.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

