Ultrastructural modifications induced by SARS-CoV-2 in Vero cells: a kinetic analysis of viral factory formation, viral particle morphogenesis and virion release
- PMID: 33449149
- PMCID: PMC7809227
- DOI: 10.1007/s00018-020-03745-y
Ultrastructural modifications induced by SARS-CoV-2 in Vero cells: a kinetic analysis of viral factory formation, viral particle morphogenesis and virion release
Abstract
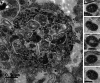
Many studies on SARS-CoV-2 have been performed over short-time scale, but few have focused on the ultrastructural characteristics of infected cells. We used TEM to perform kinetic analysis of the ultrastructure of SARS-CoV-2-infected cells. Early infection events were characterized by the presence of clusters of single-membrane vesicles and stacks of membrane containing nuclear pores called annulate lamellae (AL). A large network of host cell-derived organelles transformed into virus factories was subsequently observed in the cells. As previously described for other RNA viruses, these replication factories consisted of double-membrane vesicles (DMVs) located close to the nucleus. Viruses released at the cell surface by exocytosis harbored the typical crown of spike proteins, but viral particles without spikes were also observed in intracellular compartments, possibly reflecting incorrect assembly or a cell degradation process.
Keywords: Covid-19; Electron microscopy; SARS-CoV-2; Virus/cell interactions.
Figures







References
-
- Woo PCY, Lau SKP, Tsoi H-W, Huang Y, Poon RWS, Chu C-M, Lee RA, Luk WK, Wong GK, Wong BH, Cheng VC, Tang BS, Wu AK, Yung RW, Chen H, Guan Y, Chan KH, Yuen KY. Clinical and molecular epidemiological features of coronavirus HKU1-associated community-acquired pneumonia. J Infect Dis. 2005;192:1898–1907. doi: 10.1086/497151. - DOI - PMC - PubMed
-
- Edridge AWD, Kaczorowska J, Hoste ACR, Bakker M, Klein M, Loens K, Jebbink MF, Matser A, Kinsella CM, Rueda P, Ieven M, Goossens H, Prins M, Sastre P, Deijs M, van der Hoek L (2020) Seasonal coronavirus protective immunity is short-lasting. Nat Med. doi: 10.1038/s41591-020-1083-1 (online ahead of print) - PubMed
-
- Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, Ong S, Urbani C, Comer JA, Lim W, Rollin PE, Dowell SF, Ling AE, Humphrey CD, Shieh WJ, Guarner J, Paddock CD, Rota P, Fields B, DeRisi J, Yang JY, Cox N, Hughes JM, LeDuc JW, Bellini WJ, Anderson LJ. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

