DGK and DZHK position paper on genome editing: basic science applications and future perspective
- PMID: 33449167
- PMCID: PMC7810637
- DOI: 10.1007/s00395-020-00839-3
DGK and DZHK position paper on genome editing: basic science applications and future perspective
Abstract
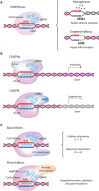
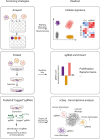
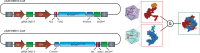
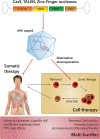
For a long time, gene editing had been a scientific concept, which was limited to a few applications. With recent developments, following the discovery of TALEN zinc-finger endonucleases and in particular the CRISPR/Cas system, gene editing has become a technique applicable in most laboratories. The current gain- and loss-of function models in basic science are revolutionary as they allow unbiased screens of unprecedented depth and complexity and rapid development of transgenic animals. Modifications of CRISPR/Cas have been developed to precisely interrogate epigenetic regulation or to visualize DNA complexes. Moreover, gene editing as a clinical treatment option is rapidly developing with first trials on the way. This article reviews the most recent progress in the field, covering expert opinions gathered during joint conferences on genome editing of the German Cardiac Society (DGK) and the German Center for Cardiovascular Research (DZHK). Particularly focusing on the translational aspect and the combination of cellular and animal applications, the authors aim to provide direction for the development of the field and the most frequent applications with their problems.
Keywords: Animal models; CRISPR/Cas; Genome editing.
Conflict of interest statement
The following conflict of interest do exist: Thomas Thum is founder and shareholder of Cardior Pharmaceuticals GmbH. Manuel Kaulich is co-founder, shareholder, and chief scientific officer of Vivlion GmbH. The following authors declare not to have a conflict of interest: Ralf P. Brandes, Anne Dueck, Stfan Engelhardt, Christian Kupatt, Maria Terese De Angelis, Matthias S. Leisegang, Ferdinand le Noble, Alessandra Moretti, Oliver J. Müller, Boris V. Skryabin, and Wolfgang Wurst.
Figures

References
-
- (2017) Gene therapy clinical trials worldwide. Provided by the Journal of Gene Medicine. http://www.abedia.com/wiley/. Accessed 14 Apr 2020
-
- (2018) NCT03655678: A Safety and Efficacy Study Evaluating CTX001 in Subjects With Transfusion-Dependent β-Thalassemia. https://clinicaltrials.gov/ct2/show/NCT03655678. Accessed 14 Apr 2020
-
- (2019) NCT03745287: A Safety and Efficacy Study Evaluating CTX001 in Subjects With Severe Sickle Cell Disease. https://clinicaltrials.gov/ct2/show/NCT03745287. Accessed 14 Apr 2020
-
- (2019) NCT03872479: Single Ascending Dose Study in Participants With LCA10. https://clinicaltrials.gov/ct2/show/NCT03872479. Accessed 14 Apr 2020
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

