Circulating Von Willebrand factor and high molecular weight multimers as markers of endothelial injury predict COVID-19 in-hospital mortality
- PMID: 33449299
- PMCID: PMC7809553
- DOI: 10.1007/s10456-020-09762-6
Circulating Von Willebrand factor and high molecular weight multimers as markers of endothelial injury predict COVID-19 in-hospital mortality
Abstract
Background: Coronavirus disease 2019 (COVID-19) is a respiratory disease associated with endotheliitis and microthrombosis.
Objectives: To correlate endothelial dysfunction to in-hospital mortality in a bi-centric cohort of COVID-19 adult patients.
Methods: Consecutive ambulatory and hospitalized patients with laboratory-confirmed COVID-19 were enrolled. A panel of endothelial biomarkers and von Willebrand factor (VWF) multimers were measured in each patient ≤ 48 h following admission.
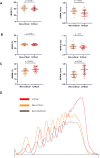
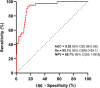
Results: Study enrolled 208 COVID-19 patients of whom 23 were mild outpatients and 189 patients hospitalized after admission. Most of endothelial biomarkers tested were found increased in the 89 critical patients transferred to intensive care unit. However, only von Willebrand factor antigen (VWF:Ag) scaled according to clinical severity, with levels significantly higher in critical patients (median 507%, IQR 428-596) compared to non-critical patients (288%, 230-350, p < 0.0001) or COVID-19 outpatients (144%, 133-198, p = 0.007). Moreover, VWF high molecular weight multimers (HMWM) were significantly higher in critical patients (median ratio 1.18, IQR 0.86-1.09) compared to non-critical patients (0.96, 1.04-1.39, p < 0.001). Among all endothelial biomarkers measured, ROC curve analysis identified a VWF:Ag cut-off of 423% as the best predictor for in-hospital mortality. The accuracy of VWF:Ag was further confirmed in a Kaplan-Meier estimator analysis and a Cox proportional Hazard model adjusted on age, BMI, C-reactive protein and D-dimer levels.
Conclusion: VWF:Ag is a relevant predictive factor for in-hospital mortality in COVID-19 patients. More than a biomarker, we hypothesize that VWF, including excess of HMWM forms, drives microthrombosis in COVID-19.
Keywords: COVID-19; Endothelial activation; Microthrombosis; Mortality; Multimers; Von Willebrand factor.
© 2021. The Author(s), under exclusive licence to Springer Nature B.V. part of Springer Nature.
Conflict of interest statement
All authors declare that they have no conflict of interest.
Figures



Comment in
-
Letter in response to: circulating von Willebrand factor and high molecular weight multimers as markers of endothelial injury predict COVID-19 in-hospital mortality.Angiogenesis. 2021 Aug;24(3):413-415. doi: 10.1007/s10456-021-09790-w. Epub 2021 Jun 8. Angiogenesis. 2021. PMID: 34101095 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

