Conditioned open-label placebo for opioid reduction after spine surgery: a randomized controlled trial
- PMID: 33449503
- PMCID: PMC8378225
- DOI: 10.1097/j.pain.0000000000002185
Conditioned open-label placebo for opioid reduction after spine surgery: a randomized controlled trial
Abstract
Placebo effects have traditionally involved concealment or deception. However, recent evidence suggests that placebo effects can also be elicited when prescribed transparently as "open-label placebos" (OLPs), and that the pairing of an unconditioned stimulus (eg, opioid analgesic) with a conditioned stimulus (eg, placebo pill) can lead to the conditioned stimulus alone reducing pain. In this randomized control trial, we investigated whether combining conditioning with an OLP (COLP) in the immediate postoperative period could reduce daily opioid use and postsurgical pain among patients recovering from spine surgery. Patients were randomized to COLP or treatment as usual, with both groups receiving unrestricted access to a typical opioid-based postoperative analgesic regimen. The generalized estimating equations method was used to assess the treatment effect of COLP on daily opioid consumption and pain during postoperative period from postoperative day (POD) 1 to POD 17. Patients in the COLP group consumed approximately 30% less daily morphine milligram equivalents compared with patients in the treatment as usual group during POD 1 to 17 (-14.5 daily morphine milligram equivalents; 95% CI: [-26.8, -2.2]). Daily worst pain scores were also lower in the COLP group (-1.0 point on the 10-point scale; 95% CI: [-2.0, -0.1]), although a significant difference was not detected in average daily pain between the groups (-0.8 point; 95% CI: [-1.7, 0.2]). These findings suggest that COLP may serve as a potential adjuvant analgesic therapy to decrease opioid consumption in the early postoperative period, without increasing pain.
Trial registration: ClinicalTrials.gov NCT04574388.
Copyright © 2021 International Association for the Study of Pain.
Conflict of interest statement
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

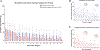


References
-
- Abrecht CR, Cornelius M, Wu A, Jamison RN, Janfaza D, Urman RD, Campbell C, Smith M, Haythornthwaite J, Edwards RR. Prediction of pain and opioid utilization in the perioperative period in patients undergoing primary knee arthroplasty: psychophysical and psychosocial factors. Pain Med 2019;20:161–71. - PMC - PubMed
-
- Ashar JK, Gordon A, Lumley M, Geuter S, Flood T, Clark J, Anderson Z, Polisky L, Carlisle J, Dimidjian S, Wager T. Open-label placebo injection for chronic back pain: a randomized controlled trial. In: P3.51, 2nd Official SIPS Conference, Society for Interdisciplinary Placebo Studies (SIPS), 2019.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

