Vigilance for Medical Products of Human Origin-Progress on the Notify Library's Global Effort to Share Information and Learning
- PMID: 33449611
- PMCID: PMC8376274
- DOI: 10.1097/TP.0000000000003589
Vigilance for Medical Products of Human Origin-Progress on the Notify Library's Global Effort to Share Information and Learning
Abstract
Background: World Health Assembly Resolution 63.22 mandated World Health Organization to facilitate Member State access to appropriate information on medical products of human origin (MPHO), including collecting data on serious adverse events and reactions. To meet this challenge, the Italian National Transplant Center, with a mandate from World Health Organization, has built and maintained an open-access searchable database of instructive records on disease transmission and other MPHO adverse occurrences.
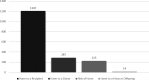
Methods: One record in the Notify Library describes a specific type of adverse occurrence in 1 type of MPHO and might be linked with 1 or multiple different references. The record inclusion criteria are that it has been reliably documented in a published article or official vigilance reporting system and that it has instructive value for the fields of transfusion, transplantation, or assisted reproduction. The selection and review of references for publication is performed by international experts who collaborate in 5 topic-specific editorial groups: infection transmission, malignancy transmission, living-donor reactions, process-related incidents, and clinical complications. New relevant references are identified through systematic searches and proactive communication by the experts.
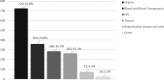
Results: The Library contains 1733 records, quoting 2632 references. Of the records, 41.8% are related to organs, 20.8% to blood and blood components, 16.5% to hematopoietic progenitor cells, 15.2% to tissues, 4.2% to reproductive tissues and cells, and 1.5% to other MPHO.
Conclusions: Notify Library is the first open-access, searchable database of systematically identified reports of disease transmission and other adverse occurrences arising from the donation and clinical application of MPHO.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors declare no funding or conflicts of interest.
Figures
Comment in
-
Donor-derived Disease-Who to Notify?Transplantation. 2021 Sep 1;105(9):1909-1910. doi: 10.1097/TP.0000000000003590. Transplantation. 2021. PMID: 33449610 No abstract available.
References
-
- Notify Library. The NOTIFY guide on vigilance and surveillance: vigilance and surveillance (V&S) of medical products of human origin (MPHO). Notify Library: the global vigilance and surveillance database for medical products of human origin. 2018. Available at https://www.notifylibrary.org/content/booklet-2018. Accessed August 24, 2020.
-
- WHO Expert Group. Expert Consensus Statement on achieving self-sufficiency in safe blood and blood products, based on voluntary non-remunerated blood donation (VNRBD). Vox Sang. 2012;103:337–342. - PubMed
-
- Fehily D, Strong DM, Minutoli D, et al. Sharing vigilance experience and knowledge globally: a preliminary overview of the Notify Library. Cells Tissues Organs. 2013;16:117–125.
-
- Strong DM. Tissue banking, biovigilance and the notify library. Cell Tissue Bank. 2018;19:187–195. - PubMed
-
- World Health Organization. WHO guiding principles on human cell, tissue and organ transplantation. Transplantation. 2010;90:229–233. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

