Design Considerations to Facilitate Clinical Radiological Evaluation of Implantable Biomedical Structures
- PMID: 33449622
- PMCID: PMC8670580
- DOI: 10.1021/acsbiomaterials.0c01439
Design Considerations to Facilitate Clinical Radiological Evaluation of Implantable Biomedical Structures
Abstract
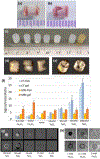
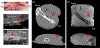
Clinical effectiveness of implantable medical devices would be improved with in situ monitoring to ensure device positioning, determine subsequent damage, measure biodegradation, and follow healing. While standard clinical imaging protocols are appropriate for diagnosing disease and injury, these protocols have not been vetted for imaging devices. This study investigated how radiologists use clinical imaging to detect the location and integrity of implanted devices and whether embedding nanoparticle contrast agents into devices can improve assessment. To mimic the variety of devices available, phantoms from hydrophobic polymer films and hydrophilic gels were constructed, with and without computed tomography (CT)-visible TaOx and magnetic resonance imaging (MRI)-visible Fe3O4 nanoparticles. Some phantoms were purposely damaged by nick or transection. Phantoms were implanted in vitro into tissue and imaged with clinical CT, MRI, and ultrasound. In a blinded study, radiologists independently evaluated whether phantoms were present, assessed the type, and diagnosed whether phantoms were damaged or intact. Radiologists identified the location of phantoms 80% of the time. However, without incorporated nanoparticles, radiologists correctly assessed damage in only 54% of cases. With an incorporated imaging agent, the percentage jumped to 86%. The imaging technique which was most useful to radiologists varied with the properties of phantoms. With benefits and drawbacks to all three imaging modalities, future implanted devices should be engineered for visibility in the modality which best fits the treated tissue, the implanted device's physical location, and the type of required information. Imaging protocols should also be tailored to best exploit the properties of the imaging agents.
Keywords: clinical imaging; contrast agent; implantable device; nanoparticles.
Figures


References
-
- Pawelec KM; Wardale RJ; Best SM; Cameron R The effects of scaffold architecture and fibrin gel addition on tendon cell phenotype. J. Mater. Sci. Mater. Med 2015, 26, 5349, DOI: 0.1007/s10856-014-5349-3. - PubMed
-
- Simons MP; Smietanski M; Bonjer HJ; Bittner R; Miserez M; Aufenacker TJ; Fitzgibbons RJ; Chowbey PK; Tran HM; Sani R; Berrevoet F; Bingener J; Bisgaard T; Bury K; Campanelli G; Chen DC; Conze J; Cuccurullo D; de Beaux AC; Eker HH; Fortelny RH; Gillion JF; van den Heuvel BJ; Hope WW; Jorgensen LN; Klinge U; Koeckerling F; Kukleta JF; Konate Il; Liem AL; Lomanto D; Loos MJA; Lopez-Cano M; Misra MC; Montgomery A; Morales-Conde S; Muysoms FE; Neibuhr H; Nordin P; Pawlak M; van Ramshorst GH; Reinpold WMJ; Sanders DL; Schouten N; Smedberg S; Simmermacher RKJ; Tumtavitikul S; van Veenendaal N; Weyhe D; Wijsmuller AR International guidelines for groin hernia management. Hernia 2018, 2(1), 1–165, DOI: 10.1007/s10029-017-1668-x. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
