Five-Year Outcomes From the Randomized, Phase III Trials CheckMate 017 and 057: Nivolumab Versus Docetaxel in Previously Treated Non-Small-Cell Lung Cancer
- PMID: 33449799
- PMCID: PMC8078445
- DOI: 10.1200/JCO.20.01605
Five-Year Outcomes From the Randomized, Phase III Trials CheckMate 017 and 057: Nivolumab Versus Docetaxel in Previously Treated Non-Small-Cell Lung Cancer
Erratum in
-
Erratum.J Clin Oncol. 2021 Apr 1;39(10):1190. doi: 10.1200/JCO.21.00546. J Clin Oncol. 2021. PMID: 33780642 Free PMC article. No abstract available.
Abstract
Purpose: Immunotherapy has revolutionized the treatment of advanced non-small-cell lung cancer (NSCLC). In two phase III trials (CheckMate 017 and CheckMate 057), nivolumab showed an improvement in overall survival (OS) and favorable safety versus docetaxel in patients with previously treated, advanced squamous and nonsquamous NSCLC, respectively. We report 5-year pooled efficacy and safety from these trials.
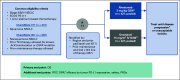
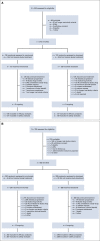
Methods: Patients (N = 854; CheckMate 017/057 pooled) with advanced NSCLC, ECOG PS ≤ 1, and progression during or after first-line platinum-based chemotherapy were randomly assigned 1:1 to nivolumab (3 mg/kg once every 2 weeks) or docetaxel (75 mg/m2 once every 3 weeks) until progression or unacceptable toxicity. The primary end point for both trials was OS; secondary end points included progression-free survival (PFS) and safety. Exploratory landmark analyses were investigated.
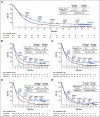
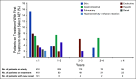
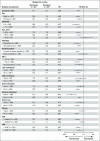
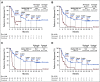
Results: After the minimum follow-up of 64.2 and 64.5 months for CheckMate 017 and 057, respectively, 50 nivolumab-treated patients and nine docetaxel-treated patients were alive. Five-year pooled OS rates were 13.4% versus 2.6%, respectively; 5-year PFS rates were 8.0% versus 0%, respectively. Nivolumab-treated patients without disease progression at 2 and 3 years had an 82.0% and 93.0% chance of survival, respectively, and a 59.6% and 78.3% chance of remaining progression-free at 5 years, respectively. Treatment-related adverse events (TRAEs) were reported in 8 of 31 (25.8%) nivolumab-treated patients between 3-5 years of follow-up, seven of whom experienced new events; one (3.2%) TRAE was grade 3, and there were no grade 4 TRAEs.
Conclusion: At 5 years, nivolumab continued to demonstrate a survival benefit versus docetaxel, exhibiting a five-fold increase in OS rate, with no new safety signals. These data represent the first report of 5-year outcomes from randomized phase III trials of a programmed death-1 inhibitor in previously treated, advanced NSCLC.
Trial registration: ClinicalTrials.gov NCT01642004 NCT01673867.
Figures




References
-
- Noone AM Howlader N Krapcho M, et al. : SEER Cancer Statistics Review, 1975–2015, National Cancer Institute, 1975-2015. https://seer.cancer.gov/csr/1975_2015/
-
- Assi HI Kamphorst AO Moukalled NM, et al. : Immune checkpoint inhibitors in advanced non-small cell lung cancer. Cancer 124:248-261, 2018 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

