Eprenetapopt (APR-246) and Azacitidine in TP53-Mutant Myelodysplastic Syndromes
- PMID: 33449813
- PMCID: PMC8099410
- DOI: 10.1200/JCO.20.02341
Eprenetapopt (APR-246) and Azacitidine in TP53-Mutant Myelodysplastic Syndromes
Abstract
Purpose: Approximately 20% of patients with TP53-mutant myelodysplastic syndromes (MDS) achieve complete remission (CR) with hypomethylating agents. Eprenetapopt (APR-246) is a novel, first-in-class, small molecule that restores wild-type p53 functions in TP53-mutant cells.
Methods: This was a phase Ib/II study to determine the safety, recommended phase II dose, and efficacy of eprenetapopt administered in combination with azacitidine in patients with TP53-mutant MDS or acute myeloid leukemia (AML) with 20%-30% marrow blasts (ClinicalTrials.gov identifier: NCT03072043).
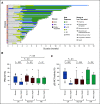
Results: Fifty-five patients (40 MDS, 11 AML, and four MDS/myeloproliferative neoplasms) with at least one TP53 mutation were treated. The overall response rate was 71% with 44% achieving CR. Of patients with MDS, 73% (n = 29) responded with 50% (n = 20) achieving CR and 58% (23/40) a cytogenetic response. The overall response rate and CR rate for patients with AML was 64% (n = 7) and 36% (n = 4), respectively. Patients with only TP53 mutations by next-generation sequencing had higher rates of CR (69% v 25%; P = .006). Responding patients had significant reductions in TP53 variant allele frequency and p53 expression by immunohistochemistry, with 21 (38%) achieving complete molecular remission (variant allele frequency < 5%). Median overall survival was 10.8 months with significant improvement in responding versus nonresponding patients by landmark analysis (14.6 v 7.5 months; P = .0005). Overall, 19/55 (35%) patients underwent allogeneic hematopoietic stem-cell transplant, with a median overall survival of 14.7 months. Adverse events were similar to those reported for azacitidine or eprenetapopt monotherapy, with the most common grade ≥ 3 adverse events being febrile neutropenia (33%), leukopenia (29%), and neutropenia (29%).
Conclusion: Combination treatment with eprenetapopt and azacitidine is well-tolerated yielding high rates of clinical response and molecular remissions in patients with TP53-mutant MDS and oligoblastic AML.
Conflict of interest statement
Figures


References
-
- Sasaki K Kanagal-Shamanna R Montalban-Bravo G, et al. : Impact of the variant allele frequency of ASXL1, DNMT3A, JAK2, TET2, TP53, and NPM1 on the outcomes of patients with newly diagnosed acute myeloid leukemia. Cancer 126:765-774, 2020 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

