TMEM16A channel upregulation in arterial smooth muscle cells produces vasoconstriction during diabetes
- PMID: 33449847
- PMCID: PMC7988758
- DOI: 10.1152/ajpheart.00690.2020
TMEM16A channel upregulation in arterial smooth muscle cells produces vasoconstriction during diabetes
Abstract
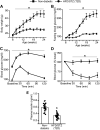
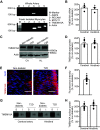
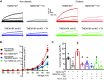
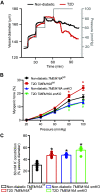
The pathological involvement of anion channels in vascular dysfunction that occurs during type 2 diabetes (T2D) is unclear. Here, we tested the hypothesis that TMEM16A, a calcium-activated chloride (Cl-) channel, contributes to modifications in arterial contractility during T2D. Our data indicate that T2D increased TMEM16A mRNA in arterial smooth muscle cells and total and surface TMEM16A protein in resistance-size cerebral and hindlimb arteries of mice. To examine vascular cell types in which TMEM16A protein increased and the functional consequences of TMEM16A upregulation during T2D, we generated tamoxifen-inducible, smooth muscle cell-specific TMEM16A knockout (TMEM16A smKO) mice. T2D increased both TMEM16A protein and Cl- current density in arterial smooth muscle cells of control (TMEM16Afl/fl) mice. In contrast, T2D did not alter arterial TMEM16A protein or Cl- current density in smooth muscle cells of TMEM16A smKO mice. Intravascular pressure stimulated greater vasoconstriction (myogenic tone) in the arteries of T2D TMEM16Afl/fl mice than in the arteries of nondiabetic TMEM16Afl/fl mice. This elevation in myogenic tone in response to T2D was abolished in the arteries of T2D TMEM16A smKO mice. T2D also reduced Akt2 protein and activity in the arteries of T2D mice. siRNA-mediated knockdown of Akt2, but not Akt1, increased arterial TMEM16A protein in nondiabetic mice. In summary, data indicate that T2D is associated with an increase in TMEM16A expression and currents in arterial smooth muscle cells that produces vasoconstriction. Data also suggest that a reduction in Akt2 function drives these pathological alterations during T2D.NEW & NOTEWORTHY We investigated the involvement of TMEM16A channels in vascular dysfunction during type 2 diabetes (T2D). TMEM16A message, protein, and currents were higher in smooth muscle cells of resistance-size arteries during T2D. Pressure stimulated greater vasoconstriction in the arteries of T2D mice that was abolished in the arteries of TMEM16A smKO mice. Akt2 protein and activity were both lower in T2D arteries, and Akt2 knockdown elevated TMEM16A protein. We propose that a decrease in Akt2 function stimulates TMEM16A expression in arterial smooth muscle cells, leading to vasoconstriction during T2D.
Keywords: Akt; TMEM16A channel; arterial contractility; smooth muscle; type 2 diabetes.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


References
-
- Centers for Disease Control and Prevention. National Diabetes Statistics Report Atlanta, Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services, 2017.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

