Mavacamten preserves length-dependent contractility and improves diastolic function in human engineered heart tissue
- PMID: 33449850
- PMCID: PMC7988756
- DOI: 10.1152/ajpheart.00325.2020
Mavacamten preserves length-dependent contractility and improves diastolic function in human engineered heart tissue
Abstract
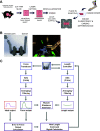
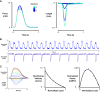
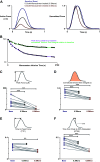
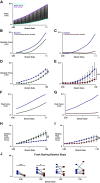
Comprehensive functional characterization of cardiac tissue includes investigation of length and load dependence. Such measurements have been slow to develop in engineered heart tissues (EHTs), whose mechanical characterizations have been limited primarily to isometric and near-isometric behaviors. A more realistic assessment of myocardial function would include force-velocity curves to characterize power output and force-length loops mimicking the cardiac cycle to characterize work output. We developed a system that produces force-velocity curves and work loops in human EHTs using an adaptive iterative control scheme. We used human EHTs in this system to perform a detailed characterization of the cardiac β-myosin specific inhibitor, mavacamten. Consistent with the clinically proposed application of this drug to treat hypertrophic cardiomyopathy, our data support the premise that mavacamten improves diastolic function through reduction of diastolic stiffness and isometric relaxation time. Meanwhile, the effects of mavacamten on length- and load-dependent muscle performance were mixed. The drug attenuated the length-dependent response at small stretch values but showed normal length dependency at longer lengths. Peak power output of mavacamten-treated EHTs showed reduced power output as expected but also shifted peak power output to a lower load. Here, we demonstrate a robust method for the generation of isotonic contraction series and work loops in engineered heart tissues using an adaptive-iterative method. This approach reveals new features of mavacamten pharmacology, including previously unappreciated effects on intrinsic myosin dynamics and preservation of Frank-Starling behavior at longer muscle lengths.NEW & NOTEWORTHY We applied innovative methods to comprehensively characterize the length and load-dependent behaviors of engineered human cardiac muscle when treated with the cardiac β-myosin specific inhibitor mavacamten, a drug on the verge of clinical implementation for hypertrophic cardiomyopathy. We find mechanistic support for the role of mavacamten in improving diastolic function of cardiac tissue and note novel effects on work and power.
Keywords: engineered heart tissues; hypertrophic cardiomyopathy; mavacamten; power.
Figures

References
-
- Cotter G, Williams SG, Vered Z, Tan LB. Role of cardiac power in heart failure. Curr Opin Cardiol 18: 215–222, 2003. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

