The development and convergence of co-pathologies in Alzheimer's disease
- PMID: 33449993
- PMCID: PMC8041349
- DOI: 10.1093/brain/awaa438
The development and convergence of co-pathologies in Alzheimer's disease
Erratum in
-
Erratum to: The development and convergence of co-pathologies in Alzheimer's disease.Brain. 2021 Aug 17;144(7):e63. doi: 10.1093/brain/awab158. Brain. 2021. PMID: 34023877 Free PMC article. No abstract available.
Abstract
Cerebral amyloid angiopathy (CAA), limbic-predominant age-related TDP-43 encephalopathy neuropathological change (LATE-NC) and Lewy bodies occur in the absence of clinical and neuropathological Alzheimer's disease, but their prevalence and severity dramatically increase in Alzheimer's disease. To investigate how plaques, tangles, age and apolipoprotein E ε4 (APOE ε4) interact with co-pathologies in Alzheimer's disease, we analysed 522 participants ≥50 years of age with and without dementia from the Center for Neurodegenerative Disease Research (CNDR) autopsy program and 1340 participants in the National Alzheimer's Coordinating Center (NACC) database. Consensus criteria were applied for Alzheimer's disease using amyloid phase and Braak stage. Co-pathology was staged for CAA (neocortical, allocortical, and subcortical), LATE-NC (amygdala, hippocampal, and cortical), and Lewy bodies (brainstem, limbic, neocortical, and amygdala predominant). APOE genotype was determined for all CNDR participants. Ordinal logistic regression was performed to quantify the effect of independent variables on the odds of having a higher stage after checking the proportional odds assumption. We found that without dementia, increasing age associated with all pathologies including CAA (odds ratio 1.63, 95% confidence interval 1.38-1.94, P < 0.01), LATE-NC (1.48, 1.16-1.88, P < 0.01), and Lewy bodies (1.45, 1.15-1.83, P < 0.01), but APOE ε4 only associated with CAA (4.80, 2.16-10.68, P < 0.01). With dementia, increasing age associated with LATE-NC (1.30, 1.15-1.46, P < 0.01), while Lewy bodies associated with younger ages (0.90, 0.81-1.00, P = 0.04), and APOE ε4 only associated with CAA (2.36, 1.52-3.65, P < 0.01). A longer disease course only associated with LATE-NC (1.06, 1.01-1.11, P = 0.01). Dementia in the NACC cohort associated with the second and third stages of CAA (2.23, 1.50-3.30, P < 0.01), LATE-NC (5.24, 3.11-8.83, P < 0.01), and Lewy bodies (2.41, 1.51-3.84, P < 0.01). Pathologically, increased Braak stage associated with CAA (5.07, 2.77-9.28, P < 0.01), LATE-NC (5.54, 2.33-13.15, P < 0.01), and Lewy bodies (4.76, 2.07-10.95, P < 0.01). Increased amyloid phase associated with CAA (2.27, 1.07-4.80, P = 0.03) and Lewy bodies (6.09, 1.66-22.33, P = 0.01). In summary, we describe widespread distributions of CAA, LATE-NC and Lewy bodies that progressively accumulate alongside plaques and tangles in Alzheimer's disease dementia. CAA interacted with plaques and tangles especially in APOE ε4 positive individuals; LATE-NC associated with tangles later in the disease course; most Lewy bodies associated with moderate to severe plaques and tangles.
Keywords: Alzheimer’s disease; CAA; TDP-43; a-synuclein; co-pathology.
© The Author(s) (2021). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
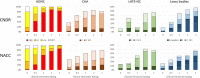
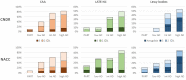
Comment in
-
Co-pathologies in Alzheimer's disease: just multiple pathologies or partners in crime?Brain. 2021 Apr 12;144(3):706-708. doi: 10.1093/brain/awab027. Brain. 2021. PMID: 33844832 No abstract available.
References
-
- Braak H, Del Tredici K, Rüb U, de Vos RAI, Jansen Steur ENH, Braak E.. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 2003; 24: 197–211. - PubMed
Publication types
MeSH terms
Grants and funding
- P50 AG005142/AG/NIA NIH HHS/United States
- P30 AG010133/AG/NIA NIH HHS/United States
- P50 AG047266/AG/NIA NIH HHS/United States
- P30 AG008017/AG/NIA NIH HHS/United States
- P30 AG010161/AG/NIA NIH HHS/United States
- P50 AG025688/AG/NIA NIH HHS/United States
- P50 AG005138/AG/NIA NIH HHS/United States
- U19 AG062418/AG/NIA NIH HHS/United States
- P50 AG047366/AG/NIA NIH HHS/United States
- P30 AG019610/AG/NIA NIH HHS/United States
- P30 AG028383/AG/NIA NIH HHS/United States
- P30 AG013854/AG/NIA NIH HHS/United States
- P30 AG053760/AG/NIA NIH HHS/United States
- P30 AG066444/AG/NIA NIH HHS/United States
- P30 AG062428/AG/NIA NIH HHS/United States
- P30 AG010124/AG/NIA NIH HHS/United States
- P50 AG023501/AG/NIA NIH HHS/United States
- P30 AG066511/AG/NIA NIH HHS/United States
- P50 AG005146/AG/NIA NIH HHS/United States
- P01 AG017586/AG/NIA NIH HHS/United States
- P30 AG062421/AG/NIA NIH HHS/United States
- P30 AG035982/AG/NIA NIH HHS/United States
- P50 AG008702/AG/NIA NIH HHS/United States
- U01 AG016976/AG/NIA NIH HHS/United States
- P30 AG008051/AG/NIA NIH HHS/United States
- P50 AG005681/AG/NIA NIH HHS/United States
- P30 AG013846/AG/NIA NIH HHS/United States
- P50 AG047270/AG/NIA NIH HHS/United States
- P30 AG062429/AG/NIA NIH HHS/United States
- P50 AG005136/AG/NIA NIH HHS/United States
- P30 AG049638/AG/NIA NIH HHS/United States
- P30 AG012300/AG/NIA NIH HHS/United States
- P30 AG062422/AG/NIA NIH HHS/United States
- P50 AG016573/AG/NIA NIH HHS/United States
- P50 AG005133/AG/NIA NIH HHS/United States
- P30 AG066509/AG/NIA NIH HHS/United States
- P30 AG062715/AG/NIA NIH HHS/United States
- P30 AG010129/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

