Biased auditory nerve central synaptopathy is associated with age-related hearing loss
- PMID: 33450070
- PMCID: PMC8197675
- DOI: 10.1113/JP281014
Biased auditory nerve central synaptopathy is associated with age-related hearing loss
Abstract
Key points: Sound information is transmitted by different subtypes of spiral ganglion neurons (SGN) from the ear to the brain. Selective damage of SGN peripheral synapses (cochlear synaptopathy) is widely recognized as one of the primary mechanisms of hearing loss, whereas the mechanisms at the SGN central synapses remain unclear. We report that different subtypes of SGN central synapses converge at different ratios onto individual target cochlear nucleus neurons with distinct physiological properties, and show biased morphological and physiological changes during age-related hearing loss (ARHL). The results reveal a new dimension in cochlear nucleus neural circuitry that systematically reassembles and processes auditory information from different SGN subtypes, which is altered during ageing and probably contributes to the development of ARHL. In addition to known cochlear synaptopathy, the present study shows that SGN central synapses are also pathologically changed during ageing, which collectively helps us better understand the structure and function of SGNs during ARHL.
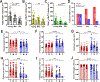
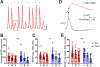
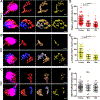
Abstract: Sound information is transmitted from the cochlea to the brain by different subtypes of spiral ganglion neurons (SGN), which show varying degrees of vulnerability under pathological conditions. Selective cochlear synaptopathy, the preferential damage of certain subtypes of SGN peripheral synapses, has been recognized as one of the main mechanisms of hearing loss. The organization and function of the auditory nerve (AN) central synapses from different subtypes of SGNs remain unclear, including how different AN synapses reassemble onto individual neurons in the cochlear nucleus, as well as how they differentially change during hearing loss. Combining immunohistochemistry with electrophysiology, we investigated the convergence pattern and subtype-specific synaptopathy of AN synapses at the endbulb of Held, as well as the response properties of their postsynaptic bushy neurons in CBA/CaJ mice of either sex under normal hearing and age-related hearing loss (ARHL). We found that calretinin-expressing (type Ia ) and non-calretinin-expressing (type Ib /Ic ) endbulbs converged along a continuum of different ratios onto individual bushy neurons with varying physiological properties. Endbulbs degenerated during ageing in parallel with ARHL. Furthermore, the degeneration was more severe in non-calretinin-expressing synapses, which correlated with a gradual decrease in bushy neuron subpopulation predominantly innervated by these inputs. These synaptic and cellular changes were profound in middle-aged mice when their hearing thresholds were still relatively normal and prior to severe ARHL. Our findings suggest that biased AN central synaptopathy and the correlated shift in cochlear nucleus neuronal composition play significant roles in weakened auditory input and altered central auditory processing during ARHL.
Keywords: age-related hearing loss; ageing; bushy neuron; cochlear nucleus; end bulb of Held; spiral ganglion neuron; synaptic physiology; synaptopathy.
© 2021 The Authors. The Journal of Physiology © 2021 The Physiological Society.
Conflict of interest statement
Competing Interests:
The authors declare no competing financial interests.
Figures








Comment in
-
Biased synaptopathy as a central mechanism of age-related hearing loss.J Physiol. 2021 Mar;599(6):1723-1724. doi: 10.1113/JP281348. Epub 2021 Feb 10. J Physiol. 2021. PMID: 33517580 No abstract available.
References
-
- Buunen TJ & Rhode WS. (1978). Responses of fibers in the cat’s auditory nerve to the cubic difference tone. The Journal of the Acoustical Society of America 64, 772–781. - PubMed
-
- Cant NB & Benson CG. (2003). Parallel auditory pathways: projection patterns of the different neuronal populations in the dorsal and ventral cochlear nuclei. Brain research bulletin 60, 457–474. - PubMed
-
- Cant NB & Morest DK. (1979). Organization of the neurons in the anterior division of the anteroventral cochlear nucleus of the cat. Light-microscopic observations. Neuroscience 4, 1909–1923. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

