A Randomized Double-Masked Phase 2a Trial to Evaluate Activity and Safety of Topical Ocular Reproxalap, a Novel RASP Inhibitor, in Dry Eye Disease
- PMID: 33450164
- PMCID: PMC8106247
- DOI: 10.1089/jop.2020.0087
A Randomized Double-Masked Phase 2a Trial to Evaluate Activity and Safety of Topical Ocular Reproxalap, a Novel RASP Inhibitor, in Dry Eye Disease
Abstract
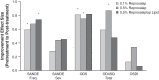
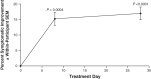
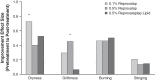
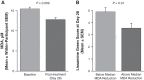
Purpose: To determine whether reproxalap, a novel reactive aldehyde species (RASP) inhibitor, is safe and effective for the treatment of the signs and symptoms of dry eye disease (DED). Methods: In a randomized double-masked parallel-group Phase 2a trial of 3 topical ocular reproxalap formulations (0.1% ophthalmic solution, 0.5% ophthalmic solution, and 0.5% lipid ophthalmic solution), 51 patients with DED were randomly assigned 1:1:1 at a single US site. Eyes were treated bilaterally 4 times daily for 28 days, and standard DED signs and symptoms were assessed at baseline and after 7 and 28 days of dosing. Tear RASP levels were assessed at baseline and at day 28. Results: The effect of treatment on DED signs and symptoms was similar across the treatment arms, and pooled data from the 28-day treatment period demonstrated significant improvement from baseline in Symptom Assessment in Dry Eye Disease score (P = 0.003), Ocular Discomfort Scale score (P < 0.0001), Ocular Discomfort Score and 4-Symptom Questionnaire overall score (P = 0.0004), Schirmer's test (P = 0.008), tear osmolarity (P = 0.003), and lissamine green total staining score (P = 0.002). Improvements in DED symptoms were evident within 1 week of therapy, and effect sizes generally approached or exceeded 0.5. No significant changes in safety measures were observed. Conclusion: The results suggest that the novel RASP inhibitor reproxalap has the potential to mitigate the signs and symptoms of DED, and may represent a new, rapidly and broadly active treatment approach for DED (NCT03162783).
Keywords: RASP inhibitor; dry eye disease; inflammation; reproxalap.
Conflict of interest statement
D.C. reports employment and stock ownership in Aldeyra Therapeutics; J.S. reports financial support from Allergan, Bausch & Lomb, EyeGate, Kala, Sun Pharmaceuticals, Tear Solutions, Inc.; consulting for and honoraria from Aldeyra Therapeutics, Allergan, Alcon, Bausch & Lomb, Clementia, Kala, Novartis, Noveome Biotherapeutics, EyeDetec, EyePoint Pharmaceuticals, EyeGate, Ocular Therapeutix, Quidel, Sun Pharmaceuticals, Tear Science, Tear Solutions, and Topivert; T.C.B. reports employment, patent interests, and stock ownership for Aldeyra Therapeutics and stock ownership in F-star Therapeutics and Evoke Pharma.
Figures
References
-
- Farrand, K.F., Fridman, M., Stillman, I.O., and Schaumberg, D.A.. Prevalence of diagnosed dry eye disease in the united states among adults aged 18 years and older. Am. J. Ophthalmol. 182:90–98, 2017 - PubMed
-
- Stapleton, F., Alves, M., Bunya, V.Y., et al. TFOS DEWS II epidemiology report. Ocul. Surf. 15:334–365, 2017 - PubMed
-
- Dana, R., Bradley, J.L., Guerin, A., Pivneva, I., Evans, A.M., and Stillman, I.O.. Comorbidities and prescribed medications in patients with or without dry eye disease: a population-based study. Am. J. Ophthalmol. 198:181–192, 2019 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
