Histone crotonylation promotes mesoendodermal commitment of human embryonic stem cells
- PMID: 33450185
- PMCID: PMC8026719
- DOI: 10.1016/j.stem.2020.12.009
Histone crotonylation promotes mesoendodermal commitment of human embryonic stem cells
Abstract
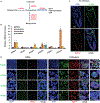
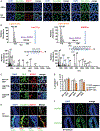
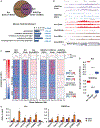
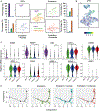
Histone crotonylation is a non-acetyl histone lysine modification that is as widespread as acetylation. However, physiological functions associated with histone crotonylation remain almost completely unknown. Here we report that histone crotonylation is crucial for endoderm differentiation. We demonstrate that key crotonyl-coenzyme A (CoA)-producing enzymes are specifically induced in endodermal cells during differentiation of human embryonic stem cells (hESCs) in vitro and in mouse embryos, where they function to increase histone crotonylation and enhance endodermal gene expression. Chemical enhancement of histone crotonylation promotes endoderm differentiation of hESCs, whereas deletion of crotonyl-CoA-producing enzymes reduces histone crotonylation and impairs meso/endoderm differentiation in vitro and in vivo. Our study uncovers a histone crotonylation-mediated mechanism that promotes endodermal commitment of pluripotent stem cells, which may have important implications for therapeutic strategies against a number of human diseases.
Keywords: crotonylation; embryogenesis; embryonic stem cells; endoderm differentiation; epigenetics; metabolic switch.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests Y.Z. is on the science advisory board of PTM Biolabs.
Figures


References
-
- Bhala A, Willi SM, Rinaldo P, Bennett MJ, Schmidt-Sommerfeld E, and Hale DE (1995). Clinical and biochemical characterization of short-chain acyl-coenzyme A dehydrogenase deficiency. J. Pediatr 126, 910–915. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

