Absence of Both Thyroid Hormone Transporters MCT8 and OATP1C1 Impairs Neural Stem Cell Fate in the Adult Mouse Subventricular Zone
- PMID: 33450189
- PMCID: PMC7878696
- DOI: 10.1016/j.stemcr.2020.12.009
Absence of Both Thyroid Hormone Transporters MCT8 and OATP1C1 Impairs Neural Stem Cell Fate in the Adult Mouse Subventricular Zone
Abstract
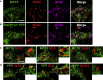
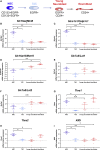
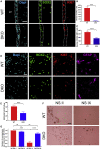
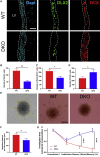
Adult neural stem cell (NSC) generation in vertebrate brains requires thyroid hormones (THs). How THs enter the NSC population is unknown, although TH availability determines proliferation and neuronal versus glial progenitor determination in murine subventricular zone (SVZ) NSCs. Mice display neurological signs of the severely disabling human disease, Allan-Herndon-Dudley syndrome, if they lack both MCT8 and OATP1C1 transporters, or MCT8 and deiodinase type 2. We analyzed the distribution of MCT8 and OATP1C1 in adult mouse SVZ. Both are strongly expressed in NSCs and at a lower level in neuronal cell precursors but not in oligodendrocyte progenitors. Next, we analyzed Mct8/Oatp1c1 double-knockout mice, where brain uptake of THs is strongly reduced. NSC proliferation and determination to neuronal fates were severely affected, but not SVZ-oligodendroglial progenitor generation. This work highlights how tight control of TH availability determines NSC function and glial-neuron cell-fate choice in adult brains.
Keywords: MCT8; OATP1C1; adult neural stem cell; olfaction; subventricular zone; thyroid hormone; thyroid hormone transporters.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Ayuso-Sacido A., Moliterno J.A., Kratovac S., Kapoor G.S., O’Rourke D.M., Holland E.C., García-Verdugo J.M., Roy N.S., Boockvar J.A. Activated EGFR signaling increases proliferation, survival, and migration and blocks neuronal differentiation in post-natal neural stem cells. J. Neurooncol. 2010;97:323–337. - PubMed
-
- Barres B.A., Lazar M.A., Raff M.C. A novel role for thyroid hormone, glucocorticoids and retinoic acid in timing oligodendrocyte development. Development. 1994;120:1097–1108. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

