Methodological quality of studies evaluating the burden of drug-resistant infections in humans due to the WHO Global Antimicrobial Resistance Surveillance System target bacteria
- PMID: 33450389
- PMCID: PMC8113024
- DOI: 10.1016/j.cmi.2021.01.004
Methodological quality of studies evaluating the burden of drug-resistant infections in humans due to the WHO Global Antimicrobial Resistance Surveillance System target bacteria
Abstract
Background: The health impact of antimicrobial resistance (AMR) has not been included in the Global Burden of Disease (GBD) report, as reliable data have been lacking. AMR burden estimates have been derived from models combining incidence and/or prevalence data from national and/or international surveillance systems and mortality estimates from clinical studies. Depending on utilized empirical data, statistical methodology and applied endpoints, the validity and reliability of results can differ substantially.
Objectives: We assessed comprehensiveness, and internal and external validity of studies estimating the clinical impact of infections caused by the priority antibiotic resistant pathogens monitored by the WHO Global Antimicrobial Resistance Surveillance System.
Data sources: Ovid MEDLINE, January 1950 to March 2019, In-Process and other non-indexed citations were searched.
Study eligibility criteria: Studies reporting mortality, length of hospital stay, duration of the disease until remission and/or death, complications, hospital re-admissions, and follow-up beyond hospital discharge were eligible.
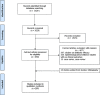
Methods: The literature was searched according to the Cochrane recommendations and reported according to Preferred Reporting Items for Systematic Reviews.
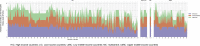
Results: Two-hundred and eighty-six studies out of 3529 were eligible. Studies derived mainly from high-income countries (215, 75%) and relied on data from retrospective (226, 79%), single-centre (201, 70%), cohort studies (243, 85%). The health impact was mostly limited to all-cause mortality (128, 45%) with heterogeneity in timing of assessment; attributable length of hospital stay was seldom adjusted for pre-infection admission time and a few studies had enough follow-up for assessing long-term sequelae. Overall, adjustment for confounding has shown a substantial improvement. Data on health state definitions and duration of diseases are generally lacking, precluding calculation of disability-adjusted life years, critical for application of the GBD study methodology.
Conclusion: Efforts to improve harmonization, representativeness, quality of AMR surveillance data and cohort studies to determine AMR attributable mortality and morbidity are urgently required. Policy makers need accurate and detailed burden estimates to inform prioritization of resource allocation, and to select the most effective intervention strategies to halt the AMR crisis.
Keywords: Antimicrobial resistance; Burden of disease; Methodology; Mortality; Surveillance.
Copyright © 2021 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures


References
-
- United Kingdom Department of Health WT . 2016. Tackling drug-resistant infections globally: final report and recommendations. The review on antimicrobial resistance.https://amr-review.org/sites/default/files/160525_Final%20paper_with%20c... Available from:
-
- Cassini A., Hogberg L.D., Plachouras D., Quattrocchi A., Hoxha A., Simonsen G.S. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis. 2019;19:56–66. - PMC - PubMed
-
- Centers for Disease Control and Prevention . 2019. Biggest threats and data.https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-re... Available from:
-
- European Centre for Disease Prevention and Control . 2020. Annual surveillance reports on antimicrobial resistance.https://www.ecdc.europa.eu/en/antimicrobial-resistance/surveillance-and-... Available from:
-
- Laxminarayan R., Duse A., Wattal C., Zaidi A.K., Wertheim H.F., Sumpradit N. Antibiotic resistance-the need for global solutions. Lancet Infect Dis. 2013;13:1057–1098. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

