A Population-Based Retrospective Study of Biliary Tract Cancers in Alberta, Canada
- PMID: 33450805
- PMCID: PMC7903272
- DOI: 10.3390/curroncol28010044
A Population-Based Retrospective Study of Biliary Tract Cancers in Alberta, Canada
Abstract
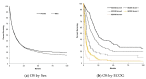
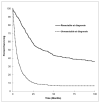
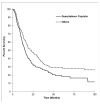
Background: Biliary tract cancers (BTC) are uncommon malignancies and are underrepresented in the literature. Methods: We performed a retrospective population-based review of adult patients with biopsy-confirmed BTC in Alberta from 2000 to 2015. Demographic data, risk factors, symptoms, treatment, and staging data were collected and analyzed. Survival analyses were completed. Results: A total of 1604 patients were included in our study, of which 766 (47.8%) were male. The median age at diagnosis was 68 (range 19-99). There were 374 (23.3%) patients with resectable tumors at diagnosis versus 597 (37.2%) with unresectable tumors. Of the patients, 380 (21.5%) received chemotherapy (CT) and 81 (5.0%) underwent radiation therapy. There was a clear trend with worsening stage and performance status associated with shorter median overall survival (OS). Ampulla of Vater tumors had the best median OS (25.69 months), while intrahepatic bile duct cancers had the worst (5.78 months). First-line palliative CT regimens included gemcitabine+cisplatin (OS 14.98 months (mo), n = 212), single agent gemcitabine (OS 12.42 mo, n = 22), capecitabine (OS 8.12 mo, n = 8), and capecitabine+gemcitabine (OS 6.93 mo, n = 13). Patients with advanced or metastatic disease who received first-line gemcitabine+cisplatin had a median OS of 11.8 months (n = 119). Conclusion: BTCs have poor survival. Worse outcomes occur in higher stage and poorer Eastern Cooperative Oncology Group (ECOG) performance status patients across all tumor subtypes. Tumor resectability at diagnosis was associated with better OS. Our study supports the use of gemcitabine+cisplatin as a combination first-line palliative CT, as patients treated in Alberta have a comparable OS to that reported in the ABC-02 phase III study.
Keywords: biliary tract neoplasms; chemotherapy; cholangiocarcinoma; gallbladder cancer; survival outcomes.
Conflict of interest statement
We have read and understood Current Oncology’s policy on conflicts of interest disclosure and declare the following interests: Eugene Batuyong has had financial interest in: Health Insurance Innovations, Adamis, VBI Vaccines, Agenus, Durect, DelMar, Avadel, Cassava, Actinium, Genmab, Onconova, Geron, iBIO, Amarin, Epizyme, Bausch, Mei Pharma, Spectrum, Acasti, TrovaGene, Bellicum, Vaxart, Cellectar Biosciences, Catalyst Biosciences, Advaxis, Exelixis, Sorrento, Leap Therapeutics, NewLink Genetics, IMV, Co-Diagnostics, Zynerba, NanoViricides, Biocryst, Altimmune, Tonix Pharmaceuticals, Rockwell Medical, Opko Health, Inovio Pharmaceuticals, Aytu Bioscience, Novavax, Correvio, Heat Biologics, Moleculin Biotech, Enzo Biochem, Diffusion Pharmaceuticals, Idera, AIM Immunotech, Capricor, Acorda, Oasis Petroleum, Ritter Pharmaceuticals, Clovis Oncology, Abeona Therapeutics, Caladrius Biosciences, Auris Medical Holding, Mesoblast, Akari, Vislink Technologies, Phio Pharmaceuticals, Karyopharm Therapeutics. Arthur Lui has received: Honoraria/Speakers’ Bureau from Astra Zeneca, Roche, MSD, Novartis. Clinical Trials Funding (To Institution) from Astra Zeneca. Vincent C. Tam has received: Honoraria from BMS, Celgene, Eisai, Ipsen, Roche. Research Funding (To Institution) from Bayer, Eisai, Ipsen. Clinical Trials Funding (To Institution) from AstraZeneca, Basilea, BMS, Boston Biomedical, Exelixis, Merck. Jennifer L. Spratlin has received: Honoraria/Advisory Boards: BMS, Celgene, Eisai, Taiho. Research Funding (To Institution) from Taiho, Merck, AstraZeneca, Celgene. Clinical Trials Funding (To Institution) from AstraZeneca, Basilea, BMS, Sanofi, Novartis, Merck.
Figures


References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

