Topical Application of Mesenchymal Stem Cell Exosomes Alleviates the Imiquimod Induced Psoriasis-Like Inflammation
- PMID: 33450859
- PMCID: PMC7828312
- DOI: 10.3390/ijms22020720
Topical Application of Mesenchymal Stem Cell Exosomes Alleviates the Imiquimod Induced Psoriasis-Like Inflammation
Abstract
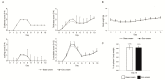
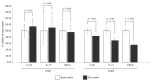
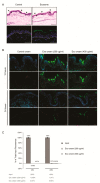
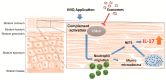
Severe psoriasis, a chronic inflammatory skin disease is increasingly being effectively managed by targeted immunotherapy but long-term immunotherapy poses health risk and loss of response. Therefore, there is a need for alternative therapy strategies. Mesenchymal stem/stromal cell (MSC) exosomes are widely known for their potent immunomodulatory properties. Here we investigated if topically applied MSC exosomes could alleviate psoriasis-associated inflammation. Topically applied fluorescent exosomes on human skin explants were confined primarily to the stratum corneum with <1% input fluorescence exiting the explant over a 24-h period. Nevertheless, topically applied MSC exosomes in a mouse model of imiquimod (IMQ) psoriasis significantly reduced IL-17 and terminal complement activation complex C5b-9 in the mouse skin. MSC exosomes were previously shown to inhibit complement activation, specifically C5b-9 complex formation through CD59. Infiltration of neutrophils into the stratum corneum is characteristic of psoriasis and neutrophils are a major cellular source of IL-17 in psoriasis through the release of neutrophil extracellular traps (NETs). We propose that topically applied MSC exosomes inhibit complement activation in the stratum corneum and this alleviates IL-17 release by NETS from neutrophils that accumulate in and beneath the stratum corneum.
Keywords: exosome; mesenchymal stem cell; psoriasis.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. S.K.L. is a founder of Paracrine Therapeutics Pte Ltd. and Vesiderm Pte Ltd.
Figures


References
-
- Menter A., Gottlieb A., Feldman S.R., Van Voorhees A.S., Leonardi C.L., Gordon K.B., Lebwohl M., Koo J.Y., Elmets C.A., Korman N.J., et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J. Am. Acad. Derm. 2008;58:826–850. doi: 10.1016/j.jaad.2008.02.039. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

