Immunotherapeutic Strategies for Neuroblastoma: Present, Past and Future
- PMID: 33450862
- PMCID: PMC7828327
- DOI: 10.3390/vaccines9010043
Immunotherapeutic Strategies for Neuroblastoma: Present, Past and Future
Abstract
Neuroblastoma is the most common extracranial pediatric solid tumor with a heterogeneous clinical course, ranging from spontaneous regression to metastatic disease and death, irrespective of intensive chemotherapeutic regimen. On the basis of several parameters, children affected by neuroblastoma are stratified into low, intermediate and high risk. At present, more than 50% of high-risk patients with metastatic spread display an overall poor long-term outcome also complicated by devastating long-term morbidities. Thus, novel and more effective therapies are desperately needed to improve lifespan of high-risk patients. In this regard, adoptive cell therapy holds great promise and several clinical trials are ongoing, demonstrating safety and tolerability, with no toxicities. Starting from the immunological and clinical features of neuroblastoma, we here discuss the immunotherapeutic approaches currently adopted for high-risk patients and different innovative therapeutic strategies currently under investigation. The latter are based on the infusion of natural killer (NK) cells, as support of consolidation therapy in addition to standard treatments, or chimeric antigen receptor (CAR) T cells directed against neuroblastoma associated antigens (e.g., disialoganglioside GD2). Finally, future perspectives of adoptive cell therapies represented by γδ T lymphocyes and CAR NK cells are envisaged.
Keywords: CAR; NK; antibodies; immunotherapy; neuroblastoma; γδ T cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
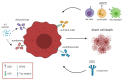
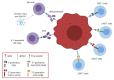
References
-
- London W.B., Castleberry R.P., Matthay K.K., Look A.T., Seeger R.C., Shimada H., Thorner P., Brodeur G., Maris J.M., Reynolds C.P., et al. Evidence for an age cutoff greater than 365 days for neuroblastoma risk group stratification in the Children’s Oncology Group. J. Clin. Oncol. 2005;23:6459–6465. doi: 10.1200/JCO.2005.05.571. - DOI - PubMed
-
- Vo K.T., Matthay K.K., Neuhaus J., London W.B., Hero B., Ambros P.F., Nakagawara A., Miniati D., Wheeler K., Pearson A.D., et al. Clinical, biologic, and prognostic differences on the basis of primary tumor site in neuroblastoma: A report from the international neuroblastoma risk group project. J. Clin. Oncol. 2014;32:3169–3176. doi: 10.1200/JCO.2014.56.1621. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

