Oxidative Stress and Lipid Mediators Modulate Immune Cell Functions in Autoimmune Diseases
- PMID: 33450863
- PMCID: PMC7828321
- DOI: 10.3390/ijms22020723
Oxidative Stress and Lipid Mediators Modulate Immune Cell Functions in Autoimmune Diseases
Abstract
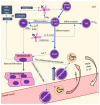
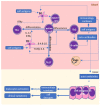
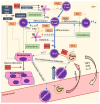
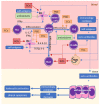
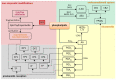
Autoimmune diseases, including psoriasis, systemic lupus erythematosus (SLE), and rheumatic arthritis (RA), are caused by a combination of environmental and genetic factors that lead to overactivation of immune cells and chronic inflammation. Since oxidative stress is a common feature of these diseases, which activates leukocytes to intensify inflammation, antioxidants could reduce the severity of these diseases. In addition to activating leukocytes, oxidative stress increases the production of lipid mediators, notably of endocannabinoids and eicosanoids, which are products of enzymatic lipid metabolism that act through specific receptors. Because the anti-inflammatory CB2 receptors are the predominant cannabinoid receptors in leukocytes, endocannabinoids are believed to act as anti-inflammatory factors that regulate compensatory mechanisms in autoimmune diseases. While administration of eicosanoids in vitro leads to the differentiation of lymphocytes into T helper 2 (Th2) cells, eicosanoids are also necessary for the different0iation of Th1 and Th17 cells. Therefore, their antagonists and/or the genetic deletion of their receptors abolish inflammation in animal models of psoriasis-RA and SLE. On the other hand, products of non-enzymatic lipid peroxidation, especially acrolein and 4-hydroxynonenal-protein adducts, mostly generated by an oxidative burst of granulocytes, may enhance inflammation and even acting as autoantigens and extracellular signaling molecules in the vicious circle of autoimmune diseases.
Keywords: ROS; endocannabinoids; immunity; lipid mediators; oxidative stress; prostaglandins; psoriasis; psoriatic arthritis; rheumatoid arthritis; systemic lupus erythematosus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Fortina A.B., Bardazzi F., Berti S., Carnevale C., Di Lernia V., El Hachem M., Neri I., Gelmetti C.M., Lora V., Mazzatenta C., et al. Treatment of severe psoriasis in children: Recommendations of an Italian expert group. Eur. J. Pediatr. 2017;176:1339–1354. doi: 10.1007/s00431-017-2985-x. - DOI - PubMed
-
- Jesus D., Matos A., Henriques C., Zen M., LaRosa M., Iaccarino L., Da Silva J.A.P., Doria A., Inês L.S. Derivation and validation of the SLE Disease Activity Score (SLE-DAS): A new SLE continuous measure with high sensitivity for changes in disease activity. Ann. Rheum. Dis. 2019;78:365–371. doi: 10.1136/annrheumdis-2018-214502. - DOI - PubMed
-
- Gonçalves R.S.G., Pereira G.A., de Andrade Lima E., Martins T.H.F., Junior J.O.A., Carvalho J.B., Mariz H.A., Dantas A.T., Duarte A.L.B.P. Validation of the Toronto Psoriatic Arthritis Screen II (ToPAS II) questionnaire in a Brazilian population. Clin. Rheumatol. 2020 doi: 10.1007/s10067-020-05509-2. - DOI - PubMed
-
- Farid E., Mumtaz M., Hajji F., Ebrahim R.A., Abdulla H., Tabbara K. T Regulatory Cells in Rheumatoid Arthritis with Reference to Anti-Citrullinated Peptide Antibody and TNF-alpha Inhibitor Therapy. Egypt J. Immunol. 2020;27:55–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

