Mg2+ Transporters in Digestive Cancers
- PMID: 33450887
- PMCID: PMC7828344
- DOI: 10.3390/nu13010210
Mg2+ Transporters in Digestive Cancers
Abstract
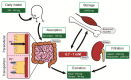
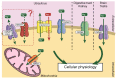
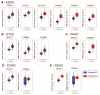
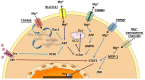
Despite magnesium (Mg2+) representing the second most abundant cation in the cell, its role in cellular physiology and pathology is far from being elucidated. Mg2+ homeostasis is regulated by Mg2+ transporters including Mitochondrial RNA Splicing Protein 2 (MRS2), Transient Receptor Potential Cation Channel Subfamily M, Member 6/7 (TRPM6/7), Magnesium Transporter 1 (MAGT1), Solute Carrier Family 41 Member 1 (SCL41A1), and Cyclin and CBS Domain Divalent Metal Cation Transport Mediator (CNNM) proteins. Recent data show that Mg2+ transporters may regulate several cancer cell hallmarks. In this review, we describe the expression of Mg2+ transporters in digestive cancers, the most common and deadliest malignancies worldwide. Moreover, Mg2+ transporters' expression, correlation and impact on patient overall and disease-free survival is analyzed using Genotype Tissue Expression (GTEx) and The Cancer Genome Atlas (TCGA) datasets. Finally, we discuss the role of these Mg2+ transporters in the regulation of cancer cell fates and oncogenic signaling pathways.
Keywords: TCGA; digestive cancers; magnesium transporters; overall survival.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

