Aberrant Dyskerin Expression Is Related to Proliferation and Poor Survival in Endometrial Cancer
- PMID: 33450922
- PMCID: PMC7828388
- DOI: 10.3390/cancers13020273
Aberrant Dyskerin Expression Is Related to Proliferation and Poor Survival in Endometrial Cancer
Abstract
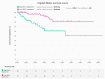
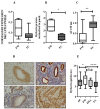
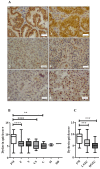
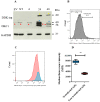
Dyskerin is a core-component of the telomerase holo-enzyme, which elongates telomeres. Telomerase is involved in endometrial epithelial cell proliferation. Most endometrial cancers (ECs) have high telomerase activity; however, dyskerin expression in human healthy endometrium or in endometrial pathologies has not been investigated yet. We aimed to examine the expression, prognostic relevance, and functional role of dyskerin in human EC. Endometrial samples from a cohort of 175 women were examined with immunohistochemistry, immunoblotting, and qPCR. The EC cells were transfected with Myc-DDK-DKC1 plasmid and the effect of dyskerin overexpression on EC cell proliferation was assessed by flow cytometry. Human endometrium expresses dyskerin (DKC1) and dyskerin protein levels are significantly reduced in ECs when compared with healthy postmenopausal endometrium. Low dyskerin immunoscores were potentially associated with worse outcomes, suggesting a possible prognostic relevance. Cancer Genome Atlas (TCGA) ECs dataset (n = 589) was also interrogated. The TCGA dataset further confirmed changes in DKC1 expression in EC with prognostic significance. Transient dyskerin overexpression had a negative effect on EC cell proliferation. Our data demonstrates a role for dyskerin in normal endometrium for the first time and confirms aberrant expression with possible prognostic relevance in EC. Interventions aimed at modulating dyskerin levels may provide novel therapeutic options in EC.
Keywords: DKC1; dyskerin; endometrial cancer; proliferation; telomerase; telomeres.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

