In Vivo Inhibition of Marek's Disease Virus in Transgenic Chickens Expressing Cas9 and gRNA against ICP4
- PMID: 33450980
- PMCID: PMC7828426
- DOI: 10.3390/microorganisms9010164
In Vivo Inhibition of Marek's Disease Virus in Transgenic Chickens Expressing Cas9 and gRNA against ICP4
Abstract
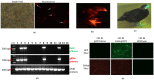
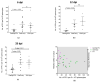
Marek's disease (MD), caused by MD herpesvirus (MDV), is an economically important disease in chickens. The efficacy of the existing vaccines against evolving virulent stains may become limited and necessitates the development of novel antiviral strategies to protect poultry from MDV strains with increased virulence. The CRISPR/Cas9 system has emerged as a powerful genome editing tool providing an opportunity to develop antiviral strategies for the control of MDV infection. Here, we characterized Tol2 transposon constructs encoding Cas9 and guide RNAs (gRNAs) specific to the immediate early infected-cell polypeptide-4 (ICP4) of MDV. We generated transgenic chickens that constitutively express Cas9 and ICP4-gRNAs (gICP4) and challenged them via intraabdominal injection of MDV-1 Woodlands strain passage-19 (p19). Transgenic chickens expressing both gRNA/Cas9 had a significantly reduced replication of MDV in comparison to either transgenic Cas9-only or the wild-type (WT) chickens. We further confirmed that the designed gRNAs exhibited sequence-specific virus interference in transgenic chicken embryo fibroblast (CEF) expressing Cas9/gICP4 when infected with MDV but not with herpesvirus of turkeys (HVT). These results suggest that CRISPR/Cas9 can be used as an antiviral approach to control MDV infection in chickens, allowing HVT to be used as a vector for recombinant vaccines.
Keywords: CRISPR/Cas9; ICP4; Marek’s disease virus; disease resilience; transgenic chicken.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Targeted Deletion of Glycoprotein B Gene by CRISPR/Cas9 Nuclease Inhibits Gallid herpesvirus Type 3 in Dually Infected Marek's Disease Virus-Transformed Lymphoblastoid Cell Line MSB-1.J Virol. 2022 Mar 23;96(6):e0202721. doi: 10.1128/jvi.02027-21. Epub 2022 Feb 2. J Virol. 2022. PMID: 35107377 Free PMC article.
-
Marek's disease virus as a CRISPR/Cas9 delivery system to defend against avian leukosis virus infection in chickens.Vet Microbiol. 2020 Mar;242:108589. doi: 10.1016/j.vetmic.2020.108589. Epub 2020 Jan 16. Vet Microbiol. 2020. PMID: 32122593
-
Effect of diluting Marek's disease vaccines on the outcomes of Marek's disease virus infection when challenged with highly virulent Marek's disease viruses.Avian Dis. 2011 Jun;55(2):263-72. doi: 10.1637/9579-101510-Reg.1. Avian Dis. 2011. PMID: 21793444 Clinical Trial.
-
History of the First-Generation Marek's Disease Vaccines: The Science and Little-Known Facts.Avian Dis. 2016 Dec;60(4):715-724. doi: 10.1637/11429-050216-Hist. Avian Dis. 2016. PMID: 27902902 Review.
-
Use of Marek's disease vaccines: could they be driving the virus to increasing virulence?Expert Rev Vaccines. 2005 Feb;4(1):77-88. doi: 10.1586/14760584.4.1.77. Expert Rev Vaccines. 2005. PMID: 15757475 Review.
Cited by
-
Harnessing Intronic microRNA Structures to Improve Tolerance and Expression of shRNAs in Animal Cells.Methods Protoc. 2022 Feb 10;5(1):18. doi: 10.3390/mps5010018. Methods Protoc. 2022. PMID: 35200534 Free PMC article.
-
Application of CRISPR/Cas gene editing for infectious disease control in poultry.Open Life Sci. 2025 May 20;20(1):20251095. doi: 10.1515/biol-2025-1095. eCollection 2025. Open Life Sci. 2025. PMID: 40417002 Free PMC article. Review.
-
Genetic resilience in chickens against bacterial, viral and protozoal pathogens.Front Vet Sci. 2022 Nov 10;9:1032983. doi: 10.3389/fvets.2022.1032983. eCollection 2022. Front Vet Sci. 2022. PMID: 36439341 Free PMC article. Review.
-
In Vitro Inhibition of Influenza Virus Using CRISPR/Cas13a in Chicken Cells.Methods Protoc. 2021 Jun 8;4(2):40. doi: 10.3390/mps4020040. Methods Protoc. 2021. PMID: 34201194 Free PMC article.
-
A Special Issue on Marek's Disease Virus-The Editors' View.Microorganisms. 2023 Mar 21;11(3):805. doi: 10.3390/microorganisms11030805. Microorganisms. 2023. PMID: 36985378 Free PMC article.
References
-
- Morrow C., Fehler F. Marek’s Disease: A Worldwide Problem. In: Davison F., Nair V., editors. Marek’s Disease: An Evolving Problem. Elsevier Academic Press; London, UK: 2004. pp. 49–61.
-
- Schat K.A., Nair V. Marek’s disease. In: Swayne D.E., Glisson J.R., McDougald L.R., Nolan L.K., Suarez D.L., Nair V.L., editors. Diseases of Poultry. Wiley-Blackwell; Ames, IA, USA: 2013. pp. 515–552.
-
- Witter R.L., Nazerian K., Purchase H.G., Burgoyne G.H. Isolation from turkeys of a cell-associated herpesvirus antigenically related to Marek’s disease virus. Am. J. Vet. Res. 1970;31:525–538. - PubMed
-
- Schat K.A. Marek’s disease: A model for protection against herpesvirus-induced tumours. Cancer Surv. 1987;6:1–37. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

