Function of the Porcine TRPC1 Gene in Myogenesis and Muscle Growth
- PMID: 33450983
- PMCID: PMC7828378
- DOI: 10.3390/cells10010147
Function of the Porcine TRPC1 Gene in Myogenesis and Muscle Growth
Abstract
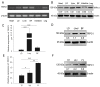
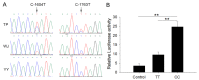
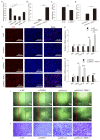
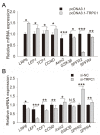
In animals, muscle growth is a quantitative trait controlled by multiple genes. Previously, we showed that the transient receptor potential channel 1 (TRPC1) gene was differentially expressed in muscle tissues between pig breeds with divergent growth traits base on RNA-seq. Here, we characterized TRPC1 expression profiles in different tissues and pig breeds and showed that TRPC1 was highly expressed in the muscle. We found two single nucleotide polymorphisms (SNPs) (C-1763T and C-1604T) in TRPC1 that could affect the promoter region activity and regulate pig growth rate. Functionally, we used RNAi and overexpression to illustrate that TRPC1 promotes myoblast proliferation, migration, differentiation, fusion, and muscle hypertrophy while inhibiting muscle degradation. These processes may be mediated by the activation of Wnt signaling pathways. Altogether, our results revealed that TRPC1 might promote muscle growth and development and plays a key role in Wnt-mediated myogenesis.
Keywords: TRPC1; muscle growth; myogenesis; porcine.
Conflict of interest statement
The authors declare that they have no conflict of interest with the contents of this article.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

