Condensing Effect of Cholesterol on hBest1/POPC and hBest1/SM Langmuir Monolayers
- PMID: 33451008
- PMCID: PMC7828479
- DOI: 10.3390/membranes11010052
Condensing Effect of Cholesterol on hBest1/POPC and hBest1/SM Langmuir Monolayers
Abstract
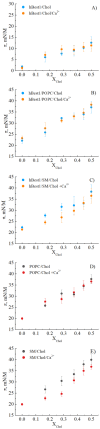
Human bestrophin-1 protein (hBest1) is a transmembrane channel associated with the calcium-dependent transport of chloride ions in the retinal pigment epithelium as well as with the transport of glutamate and GABA in nerve cells. Interactions between hBest1, sphingomyelins, phosphatidylcholines and cholesterol are crucial for hBest1 association with cell membrane domains and its biological functions. As cholesterol plays a key role in the formation of lipid rafts, motional ordering of lipids and modeling/remodeling of the lateral membrane structure, we examined the effect of different cholesterol concentrations on the surface tension of hBest1/POPC (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine) and hBest1/SM Langmuir monolayers in the presence/absence of Ca2+ ions using surface pressure measurements and Brewster angle microscopy studies. Here, we report that cholesterol: (1) has negligible condensing effect on pure hBest1 monolayers detected mainly in the presence of Ca2+ ions, and; (2) induces a condensing effect on composite hBest1/POPC and hBest1/SM monolayers. These results offer evidence for the significance of intermolecular protein-lipid interactions for the conformational dynamics of hBest1 and its biological functions as multimeric ion channel.
Keywords: Langmuir monolayers; POPC; cholesterol; condensing effect; hBest1; sphingomyelin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Grants and funding
- KP-06-N23/7, 18.12.2018/Bulgarian National Science Fund
- National Program for Research "Young Scientists and Postdoctoral Students" (РМС № 577 / 17.08.2018)/Bulgarian Ministry of Education and Science
- DO1-275/16.12.2019 "INFRAACT" of Bulgarian NRRI 2017-2023/Bulgarian Ministry of Education and Science
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

